a) G
b) The ionic radius of I is bigger than that of E since I has more energy levels than E
johnmulu answered the question on April 7, 2017 at 12:13
- The table below shows behavior of metals R, X, Y and Z. Study it and answer the questions that(Solved)
The table below shows behavior of metals R, X, Y and Z. Study it and answer the questions that follow:

a) Arrange the metals in the order of reactivity starting with the most reactive.
b) Name a metal which is likely to be: (i) X (ii) Y
Date posted: April 7, 2017. Answers (1)
- a) What is meant by the term radial?
b) The table below contains atoms that form common radicals. Complete the table to show radicals formed from various atoms
(Solved)
a) What is meant by the term radial?
b) The table below contains atoms that form common radicals. Complete the table to show radicals formed from various atoms
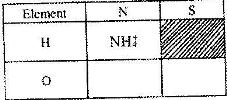
Date posted: April 7, 2017. Answers (1)
- Use the part of the periodic table given below to answer the questions that follow. (letters are not the actual symbols of elements.)(Solved)
Use the part of the periodic table given below to answer the questions that follow. (letters are not the actual symbols of elements.)
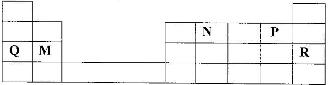
a) Identify the element that forms giant covalent structures.
b) Identify one element that does not form compounds
c) Write the formula for the nitride of M.
Date posted: April 7, 2017. Answers (1)
- The table below gives the number of electrons, protons and neutrons in substances X, Y and Z. Study it and answer the questions that follow.(Solved)
The table below gives the number of electrons, protons and neutrons in substances X, Y and Z. Study it and answer the questions that follow.

a) Which letter represents an ion?
b) Which of the substances are isotopes? Give reason.
Date posted: April 7, 2017. Answers (1)
- The table below shows the number of valence electrons of the element P, Q and R.(Solved)
The table below shows the number of valence electrons of the element P, Q and R.

a) Explain why P and R would not be expected to form a compound.
b) Write an equation to show the effect of heat on the carbonate of R.
c) Write the formula for the most stable ion of Q
Date posted: April 7, 2017. Answers (1)
- The table below shows the relative atomic masses and the percentage abudance of the isotopes L1 and L2 of element L.(Solved)
The table below shows the relative atomic masses and the percentage abudance of the isotopes L1 and L2 of element L.

Calculate the relative atomic mass of element L.
Date posted: April 7, 2017. Answers (1)
- Use the information in the table below to answer the questions that follow.
(The letters do not represent the actual symbols of the elements)
(Solved)
Use the information in the table below to answer the questions that follow.
(The letters do not represent the actual symbols of the elements)

a) Which two letters represents the same element? Give reason.
b) Give the number of neutrons in an atom of element D.
Date posted: April 7, 2017. Answers (1)
- Complete the table below.(Solved)
Complete the table below.

Date posted: April 7, 2017. Answers (1)
- The set up below was used to investigate the reaction between dry hydrogen gas and copper (II) oxide.(Solved)
The set up below was used to investigate the reaction between dry hydrogen gas and copper (II) oxide.

a) Name substance A.
b) State the observation made in the combustion tube.
c) Explain the observation stated in (b) above.
Date posted: April 7, 2017. Answers (1)
- The set up below was used to investigate the reaction between dry hydrogen gas and copper (II) oxide.(Solved)
The set up below was used to investigate the reaction between dry hydrogen gas and copper (II) oxide.

a) Name substance A.
b) State the observation made in the combustion tube.
c) Explain the observation stated in (b) above.
Date posted: April 7, 2017. Answers (1)
- The set-up below was used to investigate the products of burning biogas (methane). Study it and answer the questions that follow.(Solved)
The set-up below was used to investigate the products of burning biogas (methane). Study it and answer the questions that follow.

a) What product will be formed in test-tube Y?
b) State and explain the observations which will be made in Z.
Date posted: April 7, 2017. Answers (1)
- A student used the set up shown in the diagram below in order to study the reactions of some metals with steam. The experiment was carried out for ten minutes.(Solved)
A student used the set up shown in the diagram below in order to study the reactions of some metals with steam. The experiment was carried out for ten minutes.

a) What observation would be made if gas F is ignited?
b) When the experiment was repeated using iron powder instead of magnesium ribbon, very little gas F was obtained.
i) Give a reason for this observation.
ii) What change in the conditions of the experiment should the student have made in order to increase the volume of gas F produced?
Date posted: April 7, 2017. Answers (1)
- An experiment was set up as shown in the diagram below:(Solved)
An experiment was set up as shown in the diagram below:

a) Identify substance D.
b) Describe how the other product of the burning candle could be prevented from getting into the environment
Date posted: April 7, 2017. Answers (1)
- In laboratory experiment hydrogen gas was passed over heated copper (II) oxide as shown in the diagram below.(Solved)
In laboratory experiment hydrogen gas was passed over heated copper (II) oxide as shown in the diagram below.

Describe a chemical test that can be used to identify the product E.
Date posted: April 7, 2017. Answers (1)
- Study the diagram below and answer the question that follows(Solved)
Study the diagram below and answer the question that follows

Describe one chemical test that can be carried out to identify substance S.
Date posted: April 7, 2017. Answers (1)
- a) A candle wax is mainly a compound consisting of two elements. Name the two elements
b) The set-up below was used to investigate the burning of a candle. Study it and answer the questions that follow.
(Solved)
a) A candle wax is mainly a compound consisting of two elements. Name the two elements
b) The set-up below was used to investigate the burning of a candle. Study it and answer the questions that follow.

i) What would happen to the burning candle if the pump was turned off?
ii) State and explain the changes that are likely to occur in tube N by the end of the experiment.
iii) Name two gases that come out through tube M.
iv) What is the purpose of calcium chloride in tube L?
v) Name another substance that could be used in the place of calcium oxide in tube N.
Date posted: April 7, 2017. Answers (1)
- Study the set-up below and answer the questions that follow(Solved)
Study the set-up below and answer the questions that follow

a) Write an equation for the reaction, which takes place in the combustion tube.
b) What property of gas Z allows it to be collected as shown in the diagram?
Date posted: April 7, 2017. Answers (1)
- A student set up the experiment below to collect gas K. The gas K. The glass wool was heated before heating the zinc powder.(Solved)
A student set up the experiment below to collect gas K. The gas K. The glass wool was heated before heating the zinc powder.

a) Why was it necessary to heat the moist glass wool before heating zinc powder?
b) What would happen if the zinc powder was heated before heating the glass wool?
c) What property of gas K makes it possible for it to be collected as shown in the diagram?
Date posted: April 7, 2017. Answers (1)
- The diagram below represents a set-up that was used to react lithium with water. Study it and answer the questions that follow:
(Solved)
The diagram below represents a set-up that was used to react lithium with water. Study it and answer the questions that follow:

a) Write an equation for the reaction that takes place, given that the atomic number of lithium is 3.
b) Why would it not be advisable to use potassium in place of lithium in the above set-up?
Date posted: April 7, 2017. Answers (1)
- In an experiment, rod of metals P, Q and R were cleaned with sand paper and placed in a beaker containing water.(Solved)
In an experiment, rod of metals P, Q and R were cleaned with sand paper and placed in a beaker containing water. Another set of rod was also cleaned and placed in a beaker containing dilute acid. After placing the rods in the two liquids bubbles of gas were seen around some of the rods as shown in the diagrams below.

a) Why is it necessary to clean the rods with sand paper before dipping them into the liquids.
b) Arrange the three metals in order of their reactivity starting with the most reactive
Date posted: April 6, 2017. Answers (1)