a) Bromine: Its melting point is below room temperature and its boiling point above room temperature
b) Iodine molecules is larger than that of chlorine hence the van der Waal’s forces between the molecule
are stronger than those of chlorine.
johnmulu answered the question on April 10, 2017 at 08:38
- The diagram below represents part of the periodic table. Use it to answer the questions that follow:(Solved)
The diagram below represents part of the periodic table. Use it to answer the questions that follow:
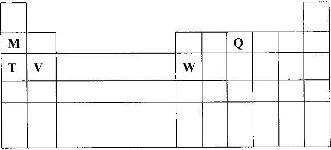
a) Write the electron arrangement for the stable ion formed by W.
b) write an equation for the reaction between V and Q.
c) How do the ionization energies of the elements M and T compare? Explain
Date posted: April 10, 2017. Answers (1)
- The ionization energies for three elements A, B and C are shown in the table below:(Solved)
The ionization energies for three elements A, B and C are shown in the table below:

Date posted: April 10, 2017. Answers (1)
- The grid below is part of the periodic table. Use it to answer the questions that follow.
(The letters are not the actual symbols of elements)
(Solved)
The grid below is part of the periodic table. Use it to answer the questions that follow.
(The letters are not the actual symbols of elements)

a) Indicate on the grid the position of an element represented by letter, V whose atomic number is 14.
b) Select a letter which represents a monoatomic gas.
c) Write an equation for reaction between Q and T.
Date posted: April 10, 2017. Answers (1)
- The table below gives some information about elements I, II, III and IV which are in the same group of the periodic table. Use the information to answer the questions that follows.(Solved)
The table below gives some information about elements I, II, III and IV which are in the same group of the periodic table. Use the information to answer the questions that follows.

State and explain the relationship between the variations in the first ionization energies and the atomic radii.
Date posted: April 10, 2017. Answers (1)
- Four metal F, G, H and J were each separately added to cold water, hot water and steam. The table below is a summary of the observations made and the formulae of the hydroxides formed.(Solved)
Four metal F, G, H and J were each separately added to cold water, hot water and steam. The table below is a summary of the observations made and the formulae of the hydroxides formed.

a) Which two elements are likely to be in the same group of the periodic table?
b) Arrange the metals in the order of their reactivity starting with the most reactive.
Date posted: April 10, 2017. Answers (1)
- The table below shows the first ionization energies of elements B and C(Solved)
The table below shows the first ionization energies of elements B and C

What do these value suggest about the reactivity of B compared to that of C?
Date posted: April 10, 2017. Answers (1)
- Study the information in the table and answer the questions that follow:(Solved)
Study the information in the table and answer the questions that follow:

Explain why: i) the ionic radius of K+ is greater than that of Na+.
ii) Mg2+ is smaller than that of Na+.
Date posted: April 10, 2017. Answers (1)
- The information in the table below relates to elements in the same group of the periodic table. Study it answer the question that follows:
(Solved)
The information in the table below relates to elements in the same group of the periodic table. Study it answer the question that follows:
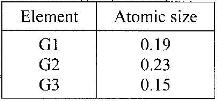
Which element has the highest ionization energy? Give a reason.
Date posted: April 10, 2017. Answers (1)
- The grid given below represents part of the periodic table. Study it and answer the questions that follow. (The letters do not represent the actual symbol of the elements)(Solved)
The grid given below represents part of the periodic table. Study it and answer the questions that follow. (The letters do not represent the actual symbol of the elements)
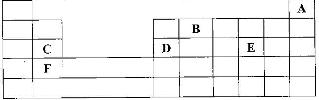
i) What name is given to the group of elements to which C and F belong?
ii) Which letter represents the element that is the least reactive?
iii) What type of bond is formed when B and E react? Explain
iv) Write the formula of the compound formed when element D and oxygen gas react
Date posted: April 10, 2017. Answers (1)
- The table below gives the energy required to remove the outermost electron for some group I elements(Solved)
The table below gives the energy required to remove the outermost electron for some group I elements
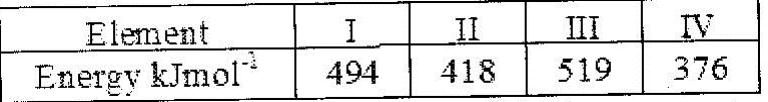
Arrange the elements in order of their reactivity starting with the most reactive.
Date posted: April 7, 2017. Answers (1)
- The grid below shows part of a periodic table. The letters do not represent the actual symbols of the elements.(Solved)
The grid below shows part of a periodic table. The letters do not represent the actual symbols of the elements.
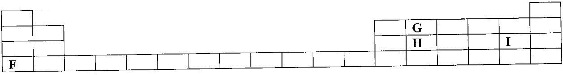
![]() a) Select the;
a) Select the;
i) Element which has the largest atomic radius.
ii) Most reactive non-metal.
Date posted: April 7, 2017. Answers (1)
- Study the table below and answer the questions that follow:(Solved)
Study the table below and answer the questions that follow:
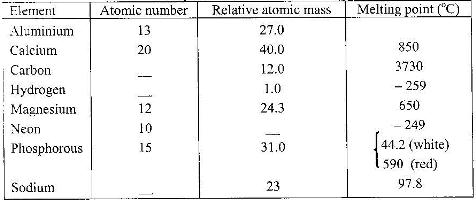
a) Complete the table by filling in the missing atomic numbers and atomic mass
b) Write the electron arrangement for the following ions: (i) Ca+ (ii) P3-
c) What is the melting point of hydrogen in Kelvin?
d) Which of the allotropes of phosphorous has a higher density? Explain.
e) The mass numbers of three isotopes of magnesium are 24, 25 and 26.
What is the mass number of the most abundant isotope of magnesium? Explain.
f) Give the formula of the compound formed between aluminium and carbon.
g) Explain the difference in the melting points of magnesium and sodium.
Date posted: April 7, 2017. Answers (1)
- The table below gives the atomic numbers of elements W, X, Y and Z. The letters do not represent the actual symbols of the elements.(Solved)
The table below gives the atomic numbers of elements W, X, Y and Z. The letters do not represent the actual symbols of the elements.
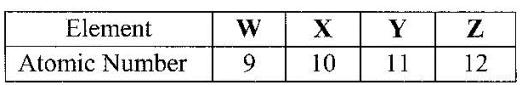
a) Which one of the elements is least reactive? Explain.
b) (i) Which two elements would react most vigorously with each other?
(ii) Give the formula of the compound formed when the elements in b (i) react.
Date posted: April 7, 2017. Answers (1)
- The table below gives information on four elements represented by letters K, L, M and N. Study it and answer the questions that follow. The letters do not represent the actual symbols of the elements.(Solved)
The table below gives information on four elements represented by letters K, L, M and N. Study it and answer the questions that follow. The letters do not represent the actual symbols of the elements.
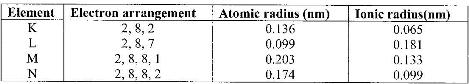
a) Which two elements have two similar properties? Explain.
b) What is the most likely formula of oxide of L?
c) Which element is a non-metal? Explain
d) Which one of the elements is the strongest reducing agent? Explain.
e) Explain why ionic radius of N is less than that of M.
f) Explain why the ionic radius of L is bigger than its atomic radius.
Date posted: April 7, 2017. Answers (1)
- The table below is part of the periodic table. The letters are not the actual symbols of the elements. Study it and answer the questions that follow.(Solved)
The table below is part of the periodic table. The letters are not the actual symbols of the elements. Study it and answer the questions that follow.
a) Select an element which is stored in paraffin in the laboratory
b) How do the ionic radii of E and I compare? Explain
Date posted: April 7, 2017. Answers (1)
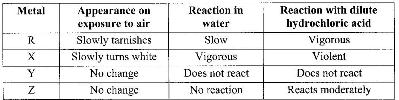
- The table below shows behavior of metals R, X, Y and Z. Study it and answer the questions that(Solved)
The table below shows behavior of metals R, X, Y and Z. Study it and answer the questions that follow:

a) Arrange the metals in the order of reactivity starting with the most reactive.
b) Name a metal which is likely to be: (i) X (ii) Y
Date posted: April 7, 2017. Answers (1)
- a) What is meant by the term radial?
b) The table below contains atoms that form common radicals. Complete the table to show radicals formed from various atoms
(Solved)
a) What is meant by the term radial?
b) The table below contains atoms that form common radicals. Complete the table to show radicals formed from various atoms
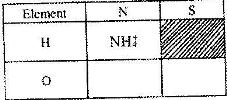
Date posted: April 7, 2017. Answers (1)
- Use the part of the periodic table given below to answer the questions that follow. (letters are not the actual symbols of elements.)(Solved)
Use the part of the periodic table given below to answer the questions that follow. (letters are not the actual symbols of elements.)
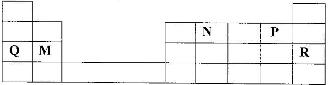
a) Identify the element that forms giant covalent structures.
b) Identify one element that does not form compounds
c) Write the formula for the nitride of M.
Date posted: April 7, 2017. Answers (1)
- The table below gives the number of electrons, protons and neutrons in substances X, Y and Z. Study it and answer the questions that follow.(Solved)
The table below gives the number of electrons, protons and neutrons in substances X, Y and Z. Study it and answer the questions that follow.

a) Which letter represents an ion?
b) Which of the substances are isotopes? Give reason.
Date posted: April 7, 2017. Answers (1)
- The table below shows the number of valence electrons of the element P, Q and R.(Solved)
The table below shows the number of valence electrons of the element P, Q and R.

a) Explain why P and R would not be expected to form a compound.
b) Write an equation to show the effect of heat on the carbonate of R.
c) Write the formula for the most stable ion of Q
Date posted: April 7, 2017. Answers (1)