a) SiH4: This is because it has a higher boiling point.
b) There’s no hydrogen bonding in CH4 and SiH4 while the hydrogen bond in
H2O is stronger than the van der waal’s forces in H2S.
johnmulu answered the question on April 10, 2017 at 09:45
- The table below gives atomic numbers of elements represented by the letters A, B, C and D.(Solved)
The table below gives atomic numbers of elements represented by the letters A, B, C and D.

Use the information to answer the questions that follow.
a) Name the type of bonding that exists in the compound formed when A and D react.
b) Select the letter which represents the best oxidizing agent. Give a reason for your answer.
Date posted: April 10, 2017. Answers (1)
- The diagram below is a section of a model of the structure of element T.(Solved)
The diagram below is a section of a model of the structure of element T.
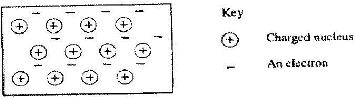
a) State the type of bonding that exists in T.
b) In which group of the periodic table does element T belong? Give a reason.
Date posted: April 10, 2017. Answers (1)
- The table below gives some information about the electrical conductivity and the likely bonding in substances N, P and Q. Complete the table by inserting the missing information in the spaces numbered I, II and III.(Solved)
The table below gives some information about the electrical conductivity and the likely bonding in substances N, P and Q. Complete the table by inserting the missing information in the spaces numbered I, II and III.

Date posted: April 10, 2017. Answers (1)
- The table below shows the properties of substances K, L, M and N.(Solved)
The table below shows the properties of substances K, L, M and N.

Date posted: April 10, 2017. Answers (1)
- Study the information in the table below and answer the questions that follow. The letters do not represent the symbols of the elements.(Solved)
Study the information in the table below and answer the questions that follow. The letters do not represent the symbols of the elements.

a) Write the electron arrangement for the ions formed by elements M and Q
b) Select an element which is
i) The most reactive non-metal
ii) A poor conductor of electricity
c) In which period of the periodic table does element R belong?
d) Element R loses its outermost electron more readily than L. Explain
Date posted: April 10, 2017. Answers (1)
- Use the information in the table below to answer the questions that follows.(Solved)
Use the information in the table below to answer the questions that follows.

Explain the trend in the molar heats of vaporization.
Date posted: April 10, 2017. Answers (1)
- Study the information given in the table below and answer the questions that follow.
The letters do not represent the actual symbols of the elements
(Solved)
Study the information given in the table below and answer the questions that follow.
The letters do not represent the actual symbols of the elements
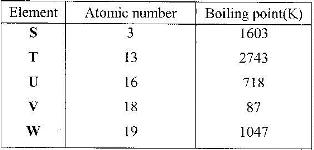
a) Select the elements which belong to the same:
i) Group
ii) Period
b) Which element
i) is in gaseous state at room temperature? Explain. (Take room temperature to be 298K)
ii) Does not form an oxide?
c) Write the
i) Formula of the nitrate of element T.
ii) Equation for the reaction between elements S and U
d) What type of bond would exists in the compound formed when U and T react? Give a reason for your answer
e) The aqueous sulphate of element W was electrolyzed using inert electrodes. Name products formed at the:
i) Cathode
ii) Anode
Date posted: April 10, 2017. Answers (1)
- The table below shows some properties of substances C, D and E. Study it and answer the questions that follow.(Solved)
The table below shows some properties of substances C, D and E. Study it and answer the questions that follow.

Select a substance:
a) With a giant molecular structure.
b) That is not likely to be an element
Date posted: April 10, 2017. Answers (1)
- Study the information below and answer the questions that follow: The letters do not represent the actual symbols of the elements.(Solved)
Study the information below and answer the questions that follow: The letters do not represent the actual symbols of the elements.
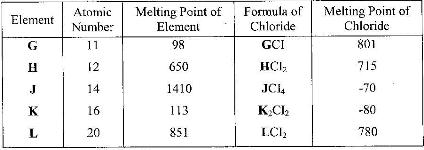
i) Which elements are metals? Give a reason.
ii) Write the formula of the compound formed when element H reacts with elements K.
iii) Explain why the melting point of J is higher than that of K.
iv) What is the oxidation state of J in its chloride.
v) How does the:
I. Melting point of fluoride of G compare with that of its chloride?
II. Reactivity of H and L with water compare? Give an explanation.
Date posted: April 10, 2017. Answers (1)
- The table below shows some properties of substance E, F, G and H(Solved)
The table below shows some properties of substance E, F, G and H

Select the substance that would be most suitable
a) For making a cooking pot
b) As a thermal insulator
Date posted: April 10, 2017. Answers (1)
- The grid below represents part of the periodic table. Study it and answer the questions that follow. The letters are not the actual symbols of the elements.(Solved)
The grid below represents part of the periodic table. Study it and answer the questions that follow. The letters are not the actual symbols of the elements.
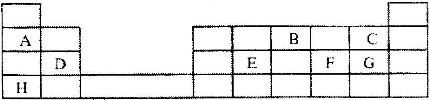
i) Select the most reactive metal. Explain.
ii) Select an element that can form an ion with a charge of 3-
iii) Select an alkaline earth metal.
iv) Which group 1 element has the highest first ionization energy? Explain
v) Element A combines with chlorine to form a chloride of A. State the most likely pH value of a solution of a chloride of A. Explain.
Date posted: April 10, 2017. Answers (1)
- The plots below were obtained when the atomic radii of some elements in groups I and II were plotted against atomic numbers.(Solved)
The plots below were obtained when the atomic radii of some elements in groups I and II were plotted against atomic numbers.
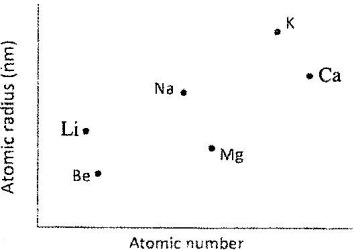
Explain:
a) The trend shown by Li, Na and K.
b) Why the atomic radii of elements Be, Mg and Ca are lower than those of Li, Na and K.
Date posted: April 10, 2017. Answers (1)
- The table below shows properties of some elements A, B, C and D which belong to the same periodic table. The letters are not the actual symbols of the elements.(Solved)
The table below shows properties of some elements A, B, C and D which belong to the same periodic table. The letters are not the actual symbols of the elements.
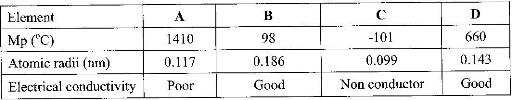
a) Arrange the elements in order they would appear in the periodic. Give a reason
b) Select the metallic element which is the better conductor of electricity. Give a reason.
Date posted: April 10, 2017. Answers (1)
- The table below gives some properties of three elements in group (VII) of the periodic table.
Study it and answer the questions that follow:
(Solved)
The table below gives some properties of three elements in group (VII) of the periodic table.
Study it and answer the questions that follow:
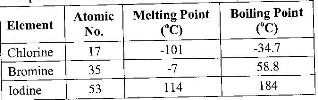
a) Which element is in liquid form at room temperature? Give a reason.
b) Explain why the boiling point of iodine is much higher than that of chlorine.
Date posted: April 10, 2017. Answers (1)
- The diagram below represents part of the periodic table. Use it to answer the questions that follow:(Solved)
The diagram below represents part of the periodic table. Use it to answer the questions that follow:
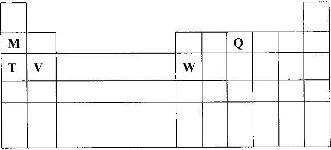
a) Write the electron arrangement for the stable ion formed by W.
b) write an equation for the reaction between V and Q.
c) How do the ionization energies of the elements M and T compare? Explain
Date posted: April 10, 2017. Answers (1)
- The ionization energies for three elements A, B and C are shown in the table below:(Solved)
The ionization energies for three elements A, B and C are shown in the table below:

Date posted: April 10, 2017. Answers (1)
- The grid below is part of the periodic table. Use it to answer the questions that follow.
(The letters are not the actual symbols of elements)
(Solved)
The grid below is part of the periodic table. Use it to answer the questions that follow.
(The letters are not the actual symbols of elements)

a) Indicate on the grid the position of an element represented by letter, V whose atomic number is 14.
b) Select a letter which represents a monoatomic gas.
c) Write an equation for reaction between Q and T.
Date posted: April 10, 2017. Answers (1)
- The table below gives some information about elements I, II, III and IV which are in the same group of the periodic table. Use the information to answer the questions that follows.(Solved)
The table below gives some information about elements I, II, III and IV which are in the same group of the periodic table. Use the information to answer the questions that follows.

State and explain the relationship between the variations in the first ionization energies and the atomic radii.
Date posted: April 10, 2017. Answers (1)
- Four metal F, G, H and J were each separately added to cold water, hot water and steam. The table below is a summary of the observations made and the formulae of the hydroxides formed.(Solved)
Four metal F, G, H and J were each separately added to cold water, hot water and steam. The table below is a summary of the observations made and the formulae of the hydroxides formed.

a) Which two elements are likely to be in the same group of the periodic table?
b) Arrange the metals in the order of their reactivity starting with the most reactive.
Date posted: April 10, 2017. Answers (1)
- The table below shows the first ionization energies of elements B and C(Solved)
The table below shows the first ionization energies of elements B and C

What do these value suggest about the reactivity of B compared to that of C?
Date posted: April 10, 2017. Answers (1)