i) Hydrogen gas
ii) Calcium hydroxide (Ca(OH)2) formed is slightly soluble in water. Therefore only a few OH- are produced in solution.
iii) It is used for testing presence of Carbon (IV) oxide (CO2)
johnmulu answered the question on April 12, 2017 at 07:08
- Carbon (II) oxide was passed over heated iron (III) oxide as shown in the diagram below.(Solved)
Carbon (II) oxide was passed over heated iron (III) oxide as shown in the diagram below.

a) Give the observation made in tube P.
b) Write the equation for the reaction which takes place in tube P.
Date posted: April 12, 2017. Answers (1)
- When steam was passed over heated charcoal as shown in the diagram below, hydrogen and carbon (II) oxide gases were formed.(Solved)
When steam was passed over heated charcoal as shown in the diagram below, hydrogen and carbon (II) oxide gases were formed.

a) Write the equation for the reaction which takes place.
b) Name two uses of carbon (II) oxide gas, which are also uses of hydrogen gas.
Date posted: April 12, 2017. Answers (1)
- The apparatus shown below was used to investigate the effect of carbon (II) oxide on copper (II) oxide.(Solved)
The apparatus shown below was used to investigate the effect of carbon (II) oxide on copper (II) oxide.

a) State the observation that was made in the combustion tube at the end of the experiment
b) Write an equation for the reaction that took place in the combustion tube.
c) why is it necessary to burn the gas coming out of tube K.
Date posted: April 12, 2017. Answers (1)
- In an experiment, carbon (IV) oxide gas was passed over heated charcoal and the gas produced collected as shown in the diagram below.(Solved)
In an experiment, carbon (IV) oxide gas was passed over heated charcoal and the gas produced collected as shown in the diagram below.
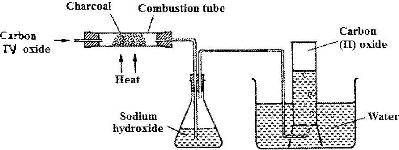
i) Write an equation for the reaction that took place in the combustion tube.
ii) Name another substance that can be used instead of sodium hydroxide
iii) Describe a simple chemical test that can be used to distinguish between carbon (IV) oxide and carbon (II) oxide.
iv) Give one use of carbon (II) oxide.
Date posted: April 10, 2017. Answers (1)
- The following diagrams show the structures of two allotropes of carbon. Study them and answer the questions that follow.(Solved)
The following diagrams show the structures of two allotropes of carbon. Study them and answer the questions that follow.

Allotrope M
(i) Name allotrope M and N
(ii) Give one use of N
(iii) Which allotrope conduct electricity? Explain.
Date posted: April 10, 2017. Answers (1)
- Study the information in the table below and answer the questions that follow.
(The letters do not represent the actual symbols of the elements.)
(Solved)
Study the information in the table below and answer the questions that follow.
(The letters do not represent the actual symbols of the elements.)

Select an element which:
a) Is likely to be in group II of the periodic table.
b) Could be used to make electric cables
c) Is likely to be graphite
Date posted: April 10, 2017. Answers (1)
- The simplified flow chart shows some of the steps in the manufacture of sodium carbonate by the Solvay process.(Solved)
The simplified flow chart shows some of the steps in the manufacture of sodium carbonate by the Solvay process.
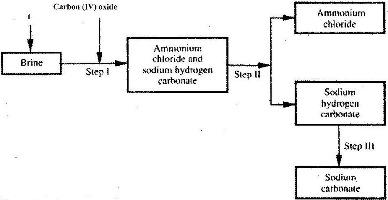
a) Identify substance L.
b) Name the process taking place in step II.
c) Write an equation for the reaction, which takes place in step III.
Date posted: April 10, 2017. Answers (1)
- The diagram below represents a charcoal burner. Study it and answer the question that follows:(Solved)
The diagram below represents a charcoal burner. Study it and answer the question that follows:

Date posted: April 10, 2017. Answers (1)
- A student investigated the effect of an electric current by passing it through some substances. The student used inert electrodes, and connected a bulb to the circuit. The table below shows the substances used and their states.(Solved)
A student investigated the effect of an electric current by passing it through some substances. The student used inert electrodes, and connected a bulb to the circuit. The table below shows the substances used and their states.

a) In which experiment did the bulb not light?
b) Explain your answer in (a) above.
Date posted: April 10, 2017. Answers (1)
- An electric current was passed through several substances and the results obtained recorded in the table below.(Solved)
An electric current was passed through several substances and the results obtained recorded in the table below.
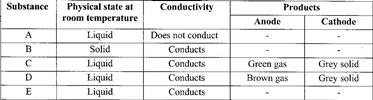
Which of these substances is likely to be:
a) Magnesium
b) Hexane
c) Lead (II) bromide
Date posted: April 10, 2017. Answers (1)
- In an experiment to investigate the conductivity of substances, a student used the set-up shown below.(Solved)
In an experiment to investigate the conductivity of substances, a student used the set-up shown below.
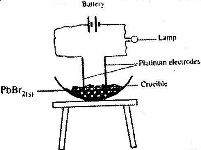
Date posted: April 10, 2017. Answers (1)
- Study the set-up below and answer the question that follows.(Solved)
Study the set-up below and answer the question that follows.
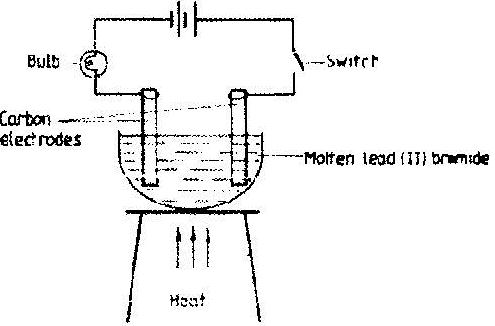
State and explain the observations that would be made when the circuit is completed
Date posted: April 10, 2017. Answers (1)
- The table below gives information about elements A1, A2, A3 and A4(Solved)
The table below gives information about elements A1, A2, A3 and A4
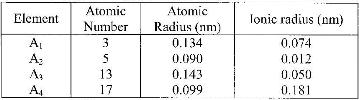
(i) In which period of the periodic table is element A2? Give a reason.
ii) Explain why the atomic radius of:
I. A1 is greater than that of A2;
II. A4 is smaller than its ionic radius.
iii) Select the element which is in the same group as A3.
Date posted: April 10, 2017. Answers (1)
- a) The scheme below shows some of the reaction of solution D. Study it and answer the questions that follows.(Solved)
a)The scheme below shows some of the reaction of solution D. Study it and answer the questions that follows.
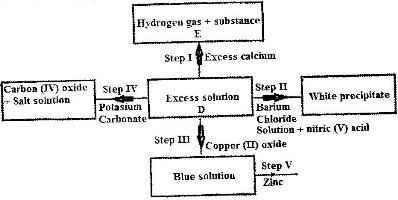
i) Give a possible cation present in solution D.
ii) What observations would be made in step V? Give a reason.
iii) Explain why the total volume of hydrogen gas produced in step 1 was found to be very low although calcium and solution D were in excess.
iv) State one use of substance E.
b) Starting with solid sodium chloride, describe how pure sample of lead (II) chloride can be prepared in the laboratory.
c) i) State a property of anhydrous calcium chloride which makes it suitable for use as a drying agent for chlorine gas.
ii) Name another substance that can be used to dry chlorine gas.
Date posted: April 10, 2017. Answers (1)
- The diagram below illustrates a method of preparing salts by direct synthesis.(Solved)
The diagram below illustrates a method of preparing salts by direct synthesis.
a) This method can be used to prepare either aluminium chloride or iron (III) chloride. Explain why it cannot be used to prepare sodium chloride.
b) Describe how a sample of sodium can be prepared in the laboratory by direct synthesis.
Date posted: April 10, 2017. Answers (1)
- a) Write an equation to show the effect of heart on the nitrate of:(Solved)
a) Write an equation to show the effect of heart on the nitrate of:
i) Potassium ii) Silver
b) The table below gives information about elements A1, A2, A3, and A4
i) In which period of the periodic table is element A2? Give reason
ii) Explain why the atomic radius of: I. A1 is greater than that of A2;
II. A4 is smaller than its ionic radius
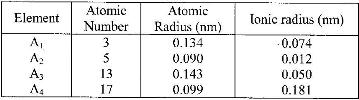
iii) Select the element which is in the same group as A3
Date posted: April 10, 2017. Answers (1)
- Study the flow chart below and answer the question that follows(Solved)
Study the flow chart below and answer the question that follows
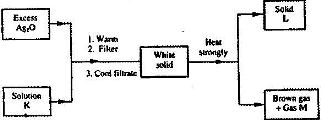
Identify
a) Solution K
b) Solid L
c) Gas M
Date posted: April 10, 2017. Answers (1)
- Use the scheme below to answer the questions that follow.(Solved)
Use the scheme below to answer the questions that follow.
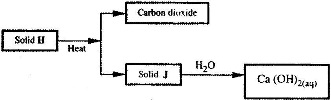
a) Identify the solids H and J
b) State one commercial use of solid J.
Date posted: April 10, 2017. Answers (1)
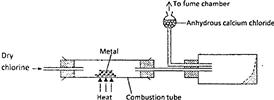
- The set-up below was used to prepare anhydrous chlorides of elements in laboratory where no fume cupboard was available. The chlorides were to be collected in flask 1.(Solved)
The set-up below was used to prepare anhydrous chlorides of elements in laboratory where no fume cupboard was available. The chlorides were to be collected in flask 1.
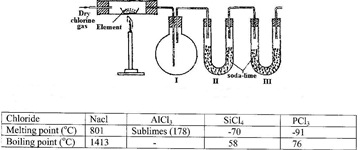
The following table shows the melting and boiling points of the chlorides that were prepared.
a) Explain why it is necessary to pass dry chlorine through the apparatus before heating each element.
b) Give two reasons why tubes II and III were filled with soda lime (solid mixture of sodium hydroxide and calcium hydroxide)
c) Explain why it would not be possible to collect any sodium chloride in flask 1
d) Name one other substance that can be used in tubes II and III
e) Write an equation for the reaction that forms phosphorous (III) chloride
f) Describe how you would separate a mixture of sodium chloride and aluminium chloride
Date posted: April 10, 2017. Answers (1)
- The grid below is part of the periodic table. Use it to answer the questions that follow. (The letters are not the actual symbols of the elements).(Solved)
The grid below is part of the periodic table. Use it to answer the questions that follow. (The letters are not the actual symbols of the elements).

a) Which is the most reactive non-metallic element shown
b) (i) Write the formula of the compound formed when element A
ii) Name the bond type in the compound formed in b (i) above.
c) i) What is the name given to the group of elements where C, G and H belong?
ii) Write the equation for the reaction that occurs when C in gaseous form is passed through a solution containing ions of element H.
d) The melting points of elements F and G are 1410oC and -101oC respectively. In terms of structure and bonding, explain why there is a large difference in the melting points of F and G.
e) D forms two oxides. Write the formula of each of the two oxides.
Date posted: April 10, 2017. Answers (1)