The cohesive force between the mercury molecules is greater than the adhesive force between the mercury and glass molecules
johnmulu answered the question on April 13, 2017 at 07:41
- The diagram in Fig. 4 shows two glass tubes of different diameters dipped in water
(Solved)
The diagram in Fig. 4 shows two glass tubes of different diameters dipped in water
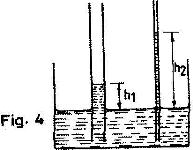
Explain why h2 is greater than h1
Date posted: April 13, 2017. Answers (1)
- Figure 2 (a) shows the initial reading of burette used to measure the volume of oil. After 50 drops of the oil were run out, the final reading was as shown in figure 2 (b)(Solved)
Figure 2 (a) shows the initial reading of burette used to measure the volume of oil. After 50 drops of the oil were run out, the final reading was as shown in figure 2 (b)
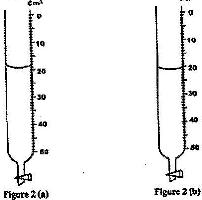
Determine the volume of one drop of oil
Date posted: April 13, 2017. Answers (1)
- Figure 1 shows a measuring cylinder containing some water(Solved)
Figure 1 shows a measuring cylinder containing some water
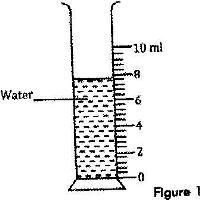
Determine the reading on the measuring cylinder, after three drops of water each of volume 0.6 cm3 are added.
Date posted: April 13, 2017. Answers (1)
- In an experiment to measure the density of a liquid, a student filled a burette with a liquid to the 0 cm 3 mark. Figure 1 shows a section of the burette showing the level of the liquid after 54.5 g of the liquid had been run out(Solved)
In an experiment to measure the density of a liquid, a student filled a burette with a liquid to the 0 cm 3 mark. Figure 1 shows a section of the burette showing the level of the liquid after 54.5 g of the liquid had been run out
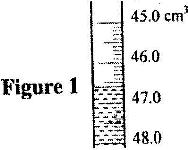
Determine the density of the liquid
Date posted: April 13, 2017. Answers (1)
- Figure 1 shows the reading on a burette after 55 drops of a liquid have been used.(Solved)
Figure 1 shows the reading on a burette after 55 drops of a liquid have been used.
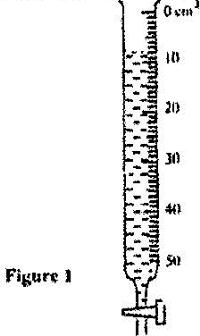
If the initial reading was at zero mark, determine the volume of one drop.
Date posted: April 13, 2017. Answers (1)
- Figure 1 shows a burette partly filed with a liquid. The burette was initially full to the mark 0. If the quantity of the liquid removed has a mass of 22 g, determine the density of the liquid(Solved)
Figure 1 shows a burette partly filed with a liquid. The burette was initially full to the mark 0. If the quantity of the liquid removed has a mass of 22 g, determine the density of the liquid
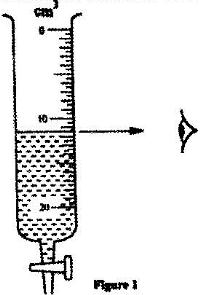
Date posted: April 13, 2017. Answers (1)
- Fig 1 shows a fencing post whose length is being measured using a strip of a measuring tape(Solved)
Fig 1 shows a fencing post whose length is being measured using a strip of a measuring tape
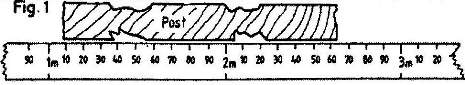
Use this information to answer the questions
a) State the accuracy of the post
b) What is the length of the post?
Date posted: April 13, 2017. Answers (1)
- Fig. 1 shows a measuring cylinder, which contains water initially at level A. A solid of mass 11 g is immersed in the water, the level rises to B.(Solved)
Fig. 1 shows a measuring cylinder, which contains water initially at level A. A solid of mass 11 g is immersed in the water, the level rises to B.

Determine the density of the solid. (Give your answer to 1 decimal point)
Date posted: April 13, 2017. Answers (1)
- What is meant by donor impurity in semiconductors? (Solved)
What is meant by donor impurity in semiconductors?
Date posted: February 22, 2017. Answers (1)
- Describe how a p-type semiconductor is formed.(Solved)
Describe how a p-type semiconductor is formed.
Date posted: February 21, 2017. Answers (1)
- It is observed that alpha particles have a lower penetrating power than beta particles. Explain this observation. (Solved)
It is observed that alpha particles have a lower penetrating power than beta particles. Explain this observation.
Date posted: February 21, 2017. Answers (1)
- Define Radioactivity?(Solved)
What is meant by Radioactivity?
Date posted: February 21, 2017. Answers (1)
- Define half-life of a radioactive material. (Solved)
Define the term half-life of a radioactive material.
Date posted: February 21, 2017. Answers (1)
- A radioactive nuclide of atomic number Z emits a beta particle and gamma rays. State the atomic number of the nuclide.(Solved)
A radioactive nuclide of atomic number Z emits a beta particle and gamma rays. State the atomic number of the nuclide.
Date posted: February 21, 2017. Answers (1)
- In a sample there are 5.12 x 1020 atoms of krypton -92 initially. If the half-life of krypton-92 is 3.0 s, determine the number of atoms that will have decayed after 6 s.(Solved)
In a sample there are 5.12 x 1020 atoms of krypton -92 initially. If the half-life of krypton-92 is 3.0 s, determine the number of atoms that will have decayed after 6 s.
Date posted: February 21, 2017. Answers (1)
- State factors that affect photoelectric emission(Solved)
State factors that affect photoelectric emission.
Date posted: February 21, 2017. Answers (1)
- State one application of thermionic emission.(Solved)
State the applications of thermionic emission.
Date posted: February 21, 2017. Answers (1)
- It is observed that when the cap of an uncharged electroscope is irradiated with light of high frequency, the leaf of electroscope rises. Explain this observation (Solved)
It is observed that when the cap of an uncharged electroscope is irradiated with light of high frequency, the leaf of electroscope rises. Explain this observation
Date posted: February 21, 2017. Answers (1)
- A photo of ultraviolet light having energy E falls on a photo emissive surface whose work function is T. Write an expression for maximum kinetic energy of the resulting photoelectron in terms of E and T. (Solved)
A photo of ultraviolet light having energy E falls on a photo emissive surface whose work function is T. Write an expression for maximum kinetic energy of the resulting photoelectron in terms of E and T.
Date posted: February 21, 2017. Answers (1)
- Light of frequency 7.5 x 1014Hz strikes a metal surface whose work function is 4.0 x 10-19 J. Determine the kinetic energy of the emitted photoelectrons. (Solved)
Light of frequency 7.5 x 1014Hz strikes a metal surface whose work function is 4.0 x 10-19 J. Determine the kinetic energy of the emitted photoelectrons. (take planks constant h = 6.63 x 10-34Js)
Date posted: February 21, 2017. Answers (1)