Sucking air reduces the air pressure inside the tube thereby making the atmospheric pressure to force the liquid up the tube
johnmulu answered the question on April 13, 2017 at 09:07
-
Figure 2 (a) and 2 (b) shows capillary tubes inserted in water and mercury respectively
(Solved)
Figure 2 (a) and 2 (b) shows capillary tubes inserted in water and mercury respectively
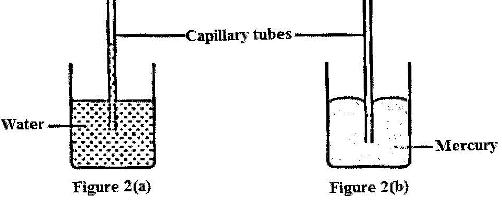
It is observed that in the water the meniscus in the capillary tube is higher than the meniscus in the beaker, while in mercury the meniscus in the capillary tube is lower than the meniscus in the beaker. Explain these observations.
Date posted:
April 13, 2017
.
Answers (1)
-
Figure 6 (a) and 6 (b) shows capillary tubes inserted in water and mercury respectively
(Solved)
Figure 6 (a) and 6 (b) shows capillary tubes inserted in water and mercury respectively
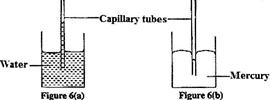
It is observed that in water the meniscus in the capillary tube is higher than the meniscus in the beaker. While in mercury the meniscus in the capillary tube is lower than the meniscus in the beaker. Explain these observations
Date posted:
April 13, 2017
.
Answers (1)
-
Figure 6 shows a small toy boat floating on water in a basin. X and Y are two points near the toy.
(Solved)
Figure 6 shows a small toy boat floating on water in a basin. X and Y are two points near the toy.
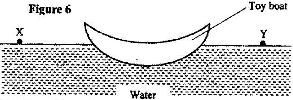
When a hot metal rod is dipped into the water at point X, the toy is observed to move towards Y. Explain this observation
Date posted:
April 13, 2017
.
Answers (1)
-
Figure 8 shows water drops on two surfaces. In figure 8 (a) the glass surface is smeared with wax while in figure 8 (b) the...
(Solved)
Figure 8 shows water drops on two surfaces. In figure 8 (a) the glass surface is smeared with wax while in figure 8 (b) the glass surface is clean.
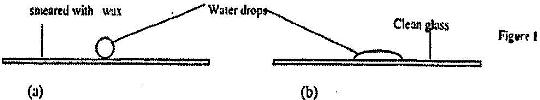
Explain the difference in the shapes of the drops
Date posted:
April 13, 2017
.
Answers (1)
-
Figure 2 (a) shows the initial reading of burette used to measure the volume of oil. After 50 drops of the oil were run out, the final reading was as shown in figure 2 (b)
(Solved)
Figure 2 (a) shows the initial reading of burette used to measure the volume of oil. After 50 drops of the oil were run out, the final reading was as shown in figure 2 (b)
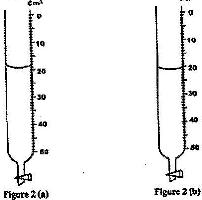
Determine the volume of one drop of oil
Date posted:
April 13, 2017
.
Answers (1)
-
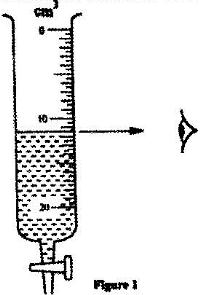
Figure 1 shows a measuring cylinder containing some water
(Solved)
Figure 1 shows a measuring cylinder containing some water
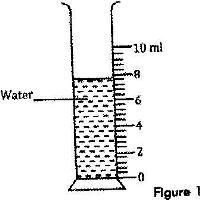
Determine the reading on the measuring cylinder, after three drops of water each of volume 0.6 cm3 are added.
Date posted:
April 13, 2017
.
Answers (1)
-
In an experiment to measure the density of a liquid, a student filled a burette with a liquid to the 0 cm 3 mark. Figure 1 shows a section of the burette showing the level of the liquid after 54.5 g of the liquid had been run out
(Solved)
In an experiment to measure the density of a liquid, a student filled a burette with a liquid to the 0 cm 3 mark. Figure 1 shows a section of the burette showing the level of the liquid after 54.5 g of the liquid had been run out
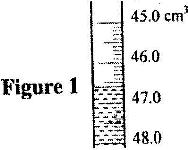
Determine the density of the liquid
Date posted:
April 13, 2017
.
Answers (1)
-
Figure 1 shows the reading on a burette after 55 drops of a liquid have been used.
(Solved)
Figure 1 shows the reading on a burette after 55 drops of a liquid have been used.
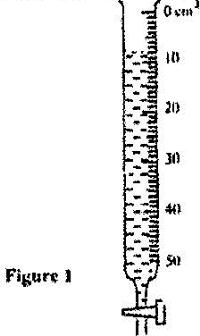
If the initial reading was at zero mark, determine the volume of one drop.
Date posted:
April 13, 2017
.
Answers (1)
-
Figure 1 shows a burette partly filed with a liquid. The burette was initially full to the mark 0. If the quantity of the liquid removed has a mass of 22 g, determine the density of the liquid
(Solved)
Figure 1 shows a burette partly filed with a liquid. The burette was initially full to the mark 0. If the quantity of the liquid removed has a mass of 22 g, determine the density of the liquid
Date posted:
April 13, 2017
.
Answers (1)
-
Fig. 1 shows a measuring cylinder, which contains water initially at level A. A solid of mass 11 g is immersed in the water, the level rises to B.
(Solved)
Fig. 1 shows a measuring cylinder, which contains water initially at level A. A solid of mass 11 g is immersed in the water, the level rises to B.

Determine the density of the solid. (Give your answer to 1 decimal point)
Date posted:
April 13, 2017
.
Answers (1)
-
In an X-ray tube it is observed that the intensity of X-rays increases when potential difference across the filament is increased.
(Solved)
In an X-ray tube it is observed that the intensity of X-rays increases when potential difference across the filament is increased. Explain this observation
Date posted:
February 20, 2017
.
Answers (1)
-
State the differences between hard X-ray and soft X-rays
(Solved)
State the differences between hard X-ray and soft X-rays.
Date posted:
February 20, 2017
.
Answers (1)
-
State and explain the effect of increasing the E.H.T in an X-ray tube on the X-rays produced.
(Solved)
State and explain the effect of increasing the E.H.T in an X-ray tube on the X-rays produced.
Date posted:
February 20, 2017
.
Answers (1)
-
Name two differences between cathode rays and electromagnetic radiations.
(Solved)
Name two differences between cathode rays and electromagnetic radiations.
Date posted:
February 20, 2017
.
Answers (1)
-
State two differences between the cathode ray tube (CRT) of a T.V. and the cathode ray oscilloscope (CRO).
(Solved)
State two differences between the cathode ray tube (CRT) of a T.V. and the cathode ray oscilloscope (CRO).
Date posted:
February 20, 2017
.
Answers (1)
-
A block of glass of mass 250 g floats in mercury. What volume of glass lies under the surface of the mercury? (Density of mercury is 13.6 x 103Kgm-3)
(Solved)
A block of glass of mass 250 g floats in mercury. What volume of glass lies under the surface of the mercury? (Density of mercury is 13.6 x 103Kgm-3)
Date posted:
February 18, 2017
.
Answers (1)
-
A block of wood weighing 2.0 N is held under water by a string attached to the bottom of a container. The tension in the string is 0.5 N
(Solved)
A block of wood weighing 2.0 N is held under water by a string attached to the bottom of a container. The tension in the string is 0.5 N. Determine the density of the wood.
Date posted:
February 18, 2017
.
Answers (1)
-
A solid copper sphere will sink in water while a hollow copper sphere of the same mass may float.
(Solved)
A solid copper sphere will sink in water while a hollow copper sphere of the same mass may float. Explain this observation.
Date posted:
February 18, 2017
.
Answers (1)
-
A long horizontal capillary tube of uniform bore sealed at one end contains dry air trapped by a drop of mercury. The length of the air column is 142 mm at 17oC.
(Solved)
A long horizontal capillary tube of uniform bore sealed at one end contains dry air trapped by a drop of mercury. The length of the air column is 142 mm at 17oC. Determine the length of the air column at 25oC.
Date posted:
February 18, 2017
.
Answers (1)
-
In verifying the pressure law of gases, the temperature and pressure of a gas are varied at constant volume.
(Solved)
In verifying the pressure law of gases, the temperature and pressure of a gas are varied at constant volume. State the condition necessary for the law to hold.
Date posted:
February 18, 2017
.
Answers (1)