Magnesium burns in air to form magnesium Oxide (MgO) and magnesium nitride (Mg3N2) reacts with water to liberate ammonia gas which turns red litmus blue
johnmulu answered the question on April 15, 2017 at 08:08
-
The diagram below shows a set-up that was used to prepare and collect a sample of nitric (V) acid.
(Solved)
The diagram below shows a set-up that was used to prepare and collect a sample of nitric (V) acid.

i) Give a reason why it is possible to separate nitric (V) acid from sulphuric (VI) acid in the set-up
ii) Name another substance that can be used instead of potassium nitrate.
iii) Give one use of nitric (V) acid
Date posted:
April 15, 2017
.
Answers (1)
-
The flow chart below shows some reactions starting with lead (II) nitrate. Study it and answer the questions that follow.
(Solved)
The flow chart below shows some reactions starting with lead (II) nitrate. Study it and answer the questions that follow.

i) State the condition necessary in step 1.
ii) Identify:
I. Reagent K.
II. Gas Q.
III. Acidic products S and R.
Date posted:
April 15, 2017
.
Answers (1)
-
Ammonium nitrate was heated as shown in the set-up below.
(Solved)
Ammonium nitrate was heated as shown in the set-up below.
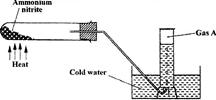
a) Identify gas A
b) State and explain a precaution that must be taken before heating is stopped.
Date posted:
April 15, 2017
.
Answers (1)
-
Ammonia gas was passed into water as shown below
(Solved)
Ammonia gas was passed into water as shown below
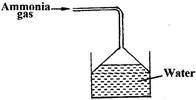
a) Explain why the pH of solution is above 7
b) What is the use of inverted tunnel?
Date posted:
April 15, 2017
.
Answers (1)
-
Ammonium nitrate was gently heated and the products collected as shown in the diagram below.
(Solved)
Ammonium nitrate was gently heated and the products collected as shown in the diagram below.
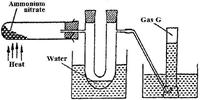
Describe one chemical and one physical method that can be used to identify gas G.
Date posted:
April 15, 2017
.
Answers (1)
-
Study the flow chart below and answer the questions that follow.
(Solved)
Study the flow chart below and answer the questions that follow.

(i) Name element M.
(ii) Why is it necessary to use excess air in step 4?
(iii) Identify gas Q.
(iv) Write an equation for the reaction in step 7
(v) Give one use of ammonia nitrate.
Date posted:
April 15, 2017
.
Answers (1)
-
A student set up the apparatus shown below to prepare ammonia gas and react it with copper (II) sulphate solution
(Solved)
A student set up the apparatus shown below to prepare ammonia gas and react it with copper (II) sulphate solution

a) Identify solution V.
b) State the observations which were made in the beaker.
Date posted:
April 15, 2017
.
Answers (1)
-
The flow chart below shows the industrial preparation of ammonia and the process used in the manufacture of some ammonium compounds. Study it and answer the questions that follow.
(Solved)
The flow chart below shows the industrial preparation of ammonia and the process used in the manufacture of some ammonium compounds. Study it and answer the questions that follow.

a) Give the name of the:
(i) Process in step 1.
(ii) Reaction that takes place in step 5.
b) State one other source of hydrogen gas apart from natural gas.
c) Explain why it is necessary to compress nitrogen and hydrogen in this process.
d) Write an equation for the reaction which takes place in step 6.
e) Name the catalyst and the reagents used in step 3.
f) Name compound Z 1
g) Give one commercial use of compound Z2
Date posted:
April 15, 2017
.
Answers (1)
-
The diagram below represents a set up that was used to obtain dry nitrogen from air. Study it and answer the questions that follow:
(Solved)
The diagram below represents a set up that was used to obtain dry nitrogen from air. Study it and answer the questions that follow:

(i) Name solid Q.
(ii) What is the purpose of sodium hydroxide?
(iii) Give the name of one impurity present in the nitrogen gas obtained.
(iv) Give a reason why liquid nitrogen is used for storage of semen for artificial insermination
Date posted:
April 15, 2017
.
Answers (1)
-
The diagram below shows a set-up that can be used to obtain nitrogen gas in an experiment.
(Solved)
The diagram below shows a set-up that can be used to obtain nitrogen gas in an experiment.

(i) Name liquid L.
(ii) What observation would be made in tube K after heating for some time?
(iii) Write an equation for the reaction that took place in tube K.
iv) At the end of experiment the pH of the water in the beaker was found to be about 10. Explain.
Date posted:
April 15, 2017
.
Answers (1)
-
Brownian motion of smoke particles can be studied by using the apparatus shown in Figure 9. To observe the motion, some smoke is enclosed in the smoke cell and then observed through the microscope.
(Solved)
Brownian motion of smoke particles can be studied by using the apparatus shown in Figure 9. To observe the motion, some smoke is enclosed in the smoke cell and then observed through the microscope.

(a) Explain the role of the smoke particles, lens and microscope in the experiment.
(b) State and explain the nature of the observed motion of the smoke particles.
(c) State what will be observed about the motion of the smoke particles if the temperature surrounding the smoke cell is raised slightly
Date posted:
April 13, 2017
.
Answers (1)
-
A can with a hole on the side is filled with water to a certain height. Water jets out as shown in figure 4 (a). A second identical can is filled with water to the same height and a block of wood floated on the water as shown in Figure 4 (b)
(Solved)
A can with a hole on the side is filled with water to a certain height. Water jets out as shown in figure 4 (a). A second identical can is filled with water to the same height and a block of wood floated on the water as shown in Figure 4 (b)

State the reason why the maximum distance of the jet, d2 is greater than d1
Date posted:
April 13, 2017
.
Answers (1)
-
Figure 1 shows the change in volume of water in a measuring cylinder when an irregular solid is immersed in it.
(Solved)
Figure 1 shows the change in volume of water in a measuring cylinder when an irregular solid is immersed in it.

Given that the mass of the solid is 567 g, determine the density of the solid in gcm-3
(Give your answer correct to 2 decimal places).
Date posted:
April 13, 2017
.
Answers (1)
-
The figure below represents the set up that was used to crack an alkane
(Solved)
The figure below represents the set up that was used to crack an alkane

a) What was the purpose of the sand?
b) After some time, a colourless gas G collected in the test-tube. Describe a chemical test and observations that would be made in order to identify the class of compounds to which gas G belongs.
Date posted:
April 12, 2017
.
Answers (1)
-
The set u below was used to prepare and collect ethane gas. Study it and answer the questions that follow.
(Solved)
The set u below was used to prepare and collect ethane gas. Study it and answer the questions that follow.

i) Name the substance T.
ii) Give the property of ethane
Date posted:
April 12, 2017
.
Answers (1)
-
a) The table below gives information about the major constituents of crude oil. Study it and answer the questions that follow.
(Solved)
a) The table below gives information about the major constituents of crude oil. Study it and answer the questions that follow.

i) Which one of the constituents of crude oil has molecules with the highest number of carbon atoms? Explain
ii) Name the process you would use to separate a mixture of petrol and diesel and explain how the separation takes place
iii) Explain why the constituent of crude oil have a range of boiling point.
Date posted:
April 12, 2017
.
Answers (1)
-
Use the information in the table below to answer the questions that follow. The letters do not represent the actual symbols of the elements
(Solved)
Use the information in the table below to answer the questions that follow. The letters do not represent the actual symbols of the elements

a) Give reasons why the melting point of:
(i) S is higher than that of R;
(ii) V is lower than of U.
b) How does the reactivity of W with chlorine compare with that of R with chlorine? Explain.
c) Write an equation for the reaction between T and excess oxygen.
d) Give one use element V.
Date posted:
April 12, 2017
.
Answers (1)
-
The grid given below represents part of the periodic table. Study it and answer the questions that follow. The letters do not represent the actual symbols of elements
(Solved)
The grid given below represents part of the periodic table. Study it and answer the questions that follow. The letters do not represent the actual symbols of elements

(i) Select a letter which represents an element that looses electrons most readily. Given a reason for your answer
(ii) Explain why the atomic radius of P is found to be smaller than that of N.
Date posted:
April 12, 2017
.
Answers (1)
-
Use the information in the table below to answer the questions that follow. The letters do not represent the actual symbols of the elements.
(Solved)
Use the information in the table below to answer the questions that follow. The letters do not represent the actual symbols of the elements.

a) Give reasons why the melting point of:
i) S is higher than that of R;
ii) V is lower than that of U.
b) How does the reactivity of W with chlorine compare with that of R with chlorine? Explain
c) Write an equation for the reaction between T and excess oxygen.
d) Give one use of element V.
Date posted:
April 12, 2017
.
Answers (1)
-
. The chart below is part of the periodic table. Study it and answer the questions that follow. (The letters are not the actual symbols of the elements)
(Solved)
The chart below is part of the periodic table. Study it and answer the questions that follow. (The letters are not the actual symbols of the elements)

i) Select the element in period three which has the shorter atomic radius. Give a reason for your answer
ii) Element F has the electronic structure, 2.8.18.4. On the chart above, indicate the position of element F.
iii) State one use of the elements of which E is a member.
iv) Write an equation to show the action of heat on the nitrate of element C.
Date posted:
April 12, 2017
.
Answers (1)