Water and glass are poor conductors of heat
johnmulu answered the question on April 15, 2017 at 09:30
- Figure 2 shows a flat bottomed flask containing some water. It is heated directly with a very hot flame. Explain why the flask is likely to crack(Solved)
Figure 2 shows a flat bottomed flask containing some water. It is heated directly with a very hot flame. Explain why the flask is likely to crack
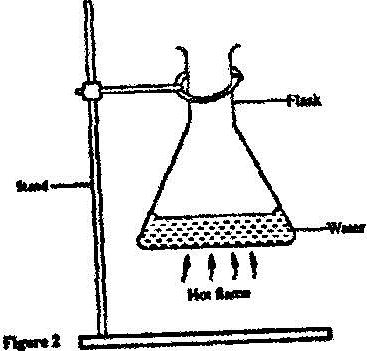
Date posted: April 15, 2017. Answers (1)
- Figure 3 shows an aluminium tube tightly stuck in a steel tube.(Solved)
Figure 3 shows an aluminium tube tightly stuck in a steel tube.

Explain how the two tubes can be separated by applying a temperature change at the same junction given that aluminium expands more than steel for the same temperature rise.
Date posted: April 15, 2017. Answers (1)
- In the set up shown in figure 4, it is observed that the level of the water initially drops before starting to rise.
(Solved)
In the set up shown in figure 4, it is observed that the level of the water initially drops before starting to rise.
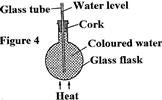
Explain the observation.
Date posted: April 15, 2017. Answers (1)
- Figure 5 shows a flask fitted with a glass tube dipped into a beaker containing water at room temperature. The cork fixing the glass tube to the flask is airtight.(Solved)
Figure 5 shows a flask fitted with a glass tube dipped into a beaker containing water at room temperature. The cork fixing the glass tube to the flask is airtight.
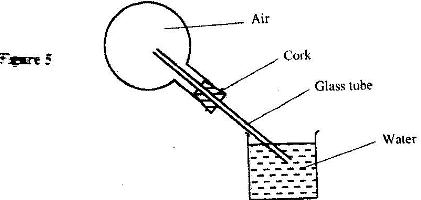
Use the information and the figure to answer the questions
a) State what is observed when ice-cold water is poured on the flask
b) Give a reason for the observation
Date posted: April 13, 2017. Answers (1)
- Figure 4 shows ma bimetallic thermometer
(Solved)
Figure 4 shows ma bimetallic thermometer
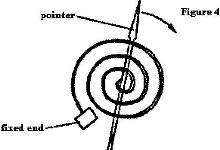
Explain how a rise in temperature causes the pointer to move in the direction shown
Date posted: April 13, 2017. Answers (1)
- Figure 10 shows a fire alarm circuit(Solved)
Figure 10 shows a fire alarm circuit

Explain how the alarm functions
Date posted: April 13, 2017. Answers (1)
- Figure 1 shows a circuit diagram for controlling the temperature of a room(Solved)
Figure 1 shows a circuit diagram for controlling the temperature of a room

i) State and explain the purpose of the bimetallic strip.
ii) Describe how the circuit controls the temperature when the switch S is closed.
Date posted: April 13, 2017. Answers (1)
- Figure 3 shows the arrangement of molecules in the three states of matter(Solved)
Figure 3 shows the arrangement of molecules in the three states of matter
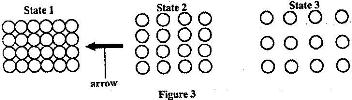
a) Name the process represented by the arrow.
b) State the reason for the arrangement of molecules in state 3.
Date posted: April 13, 2017. Answers (1)
- Figure 4 (i) shows a beaker filled with water. Some potassium permanganate was gently introduced at the bottom of the beaker at the position shown(Solved)
Figure 4 (i) shows a beaker filled with water. Some potassium permanganate was gently introduced at the bottom of the beaker at the position shown
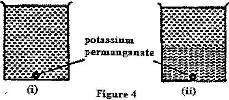
Figure 4 (ii) shows the appearance of the liquid after about 30 minutes. Explain how this appearance was caused
Date posted: April 13, 2017. Answers (1)
- Figure 3 shows two cylinders of different cross-sectional areas with a tube. The cylinder contain an incompressible fluid and are fitted with piston of cross-sectional areas 4 cm2 and 24 cm2(Solved)
Figure 3 shows two cylinders of different cross-sectional areas with a tube. The cylinder contain an incompressible fluid and are fitted with piston of cross-sectional areas 4 cm2 and 24 cm2
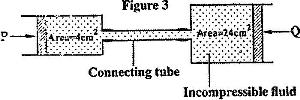
Opposing forces P and Q are applied to the pistons such that the pistons do not move. If the pressure on the smaller piston is 5 Ncm-2, determine force Q.
Date posted: April 13, 2017. Answers (1)
- Figure 2 shows some air trapped by mercury in a glass tube. The tube is inverted in a dish containing mercury.(Solved)
Figure 2 shows some air trapped by mercury in a glass tube. The tube is inverted in a dish containing mercury.
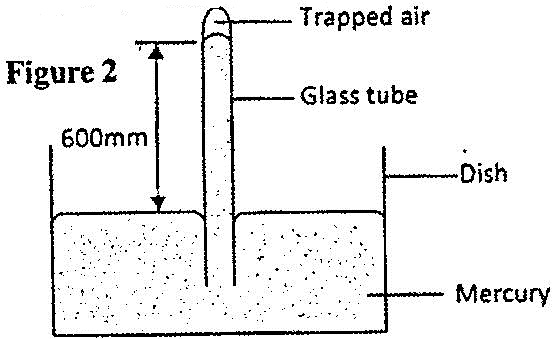
Given that the atmospheric pressure is 760 mmHg and the height of mercury column in the tube is 600 mm, determine the pressure of the air trapped in mmHg
Date posted: April 13, 2017. Answers (1)
- Figure 14 shows a lift pump.(Solved)
Figure 14 shows a lift pump.
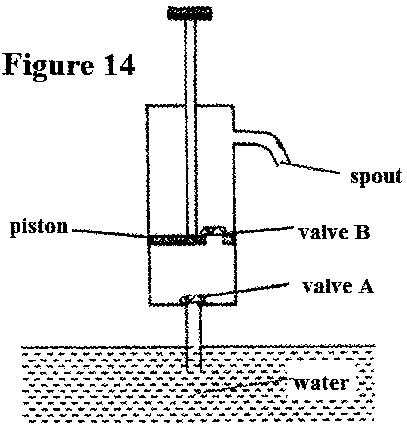
Explain why, when the piston is:
(i) pulled upwards, valve A opens while valve B closes.
(ii) Pushed downwards, valve A closes while valve B opens
Date posted: April 13, 2017. Answers (1)
- Figure 9 shows a syringe full of water. It has two identical holes A and B drilled along its cylinder. The cylinder nozzle is closed
(Solved)
Figure 9 shows a syringe full of water. It has two identical holes A and B drilled along its cylinder. The cylinder nozzle is closed
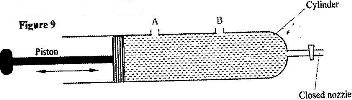
State with a reason how the speed of the jets of water from A and B compare when the piston is pushed into the cylinder.
Date posted: April 13, 2017. Answers (1)
- Figure 2 shows two cylinders containing a liquid and connected with a tight-fitting flexible tube. The cylinders are fitted with air-tight pistons A and B as shown(Solved)
Figure 2 shows two cylinders containing a liquid and connected with a tight-fitting flexible tube. The cylinders are fitted with air-tight pistons A and B as shown
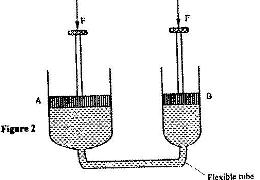
When equal forces, F, are applied on the pistons as shown it is observed that piston A moves up while B moves up while B moves down. Explain this observation
Date posted: April 13, 2017. Answers (1)
- Figure 3 shows the levels of two liquids A and B after some air has been sucked out of the tubes through the tap. Use...(Solved)
Figure 3 shows the levels of two liquids A and B after some air has been sucked out of the tubes through the tap. Use this information and the figure to answer questions
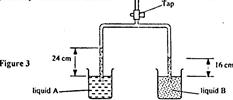
State the reason for the rise in the levels of the liquids when air is sucked from the tubes
Date posted: April 13, 2017. Answers (1)
- Figure 2 (a) and 2 (b) shows capillary tubes inserted in water and mercury respectively
(Solved)
Figure 2 (a) and 2 (b) shows capillary tubes inserted in water and mercury respectively
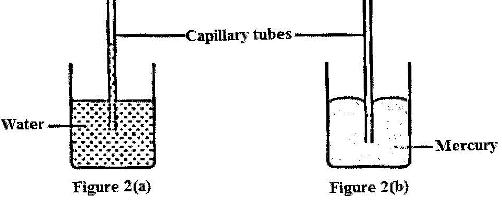
It is observed that in the water the meniscus in the capillary tube is higher than the meniscus in the beaker, while in mercury the meniscus in the capillary tube is lower than the meniscus in the beaker. Explain these observations.
Date posted: April 13, 2017. Answers (1)
- Figure 6 (a) and 6 (b) shows capillary tubes inserted in water and mercury respectively(Solved)
Figure 6 (a) and 6 (b) shows capillary tubes inserted in water and mercury respectively
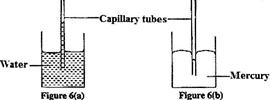
It is observed that in water the meniscus in the capillary tube is higher than the meniscus in the beaker. While in mercury the meniscus in the capillary tube is lower than the meniscus in the beaker. Explain these observations
Date posted: April 13, 2017. Answers (1)
- Figure 6 shows a small toy boat floating on water in a basin. X and Y are two points near the toy.(Solved)
Figure 6 shows a small toy boat floating on water in a basin. X and Y are two points near the toy.
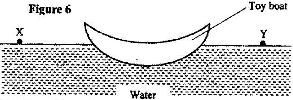
When a hot metal rod is dipped into the water at point X, the toy is observed to move towards Y. Explain this observation
Date posted: April 13, 2017. Answers (1)
- Figure 8 shows water drops on two surfaces. In figure 8 (a) the glass surface is smeared with wax while in figure 8 (b) the...(Solved)
Figure 8 shows water drops on two surfaces. In figure 8 (a) the glass surface is smeared with wax while in figure 8 (b) the glass surface is clean.
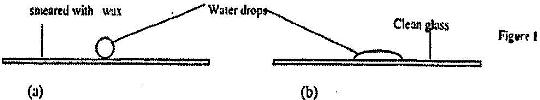
Explain the difference in the shapes of the drops
Date posted: April 13, 2017. Answers (1)
- Figure 4 shows a capillary tube placed in through mercury.(Solved)
Figure 4 shows a capillary tube placed in through mercury.
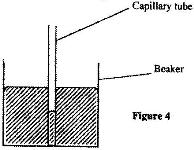
Give a reason why the level of mercury in the capillary tube is lower than in the beaker
Date posted: April 13, 2017. Answers (1)