X being black absorbs heat faster than Y. The gas in X is therefore heated - it expands pushing the water level S downwards, which causes the level in T to rise
johnmulu answered the question on April 17, 2017 at 09:10
- Figure 3 shows a piece of wood fitted into copper pipe and a piece of a paper wrapped tightly around the junction.(Solved)
Figure 3 shows a piece of wood fitted into copper pipe and a piece of a paper wrapped tightly around the junction.
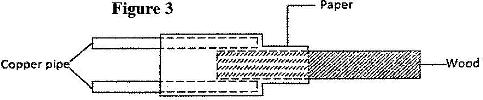
It is observed that when a flame is applied around the paper at the junction, the side of the paper around the wood burns first. Explain this observation.
Date posted: April 17, 2017. Answers (1)
- a) Figure 11 shows how an underground room was ventilated. It had two vents, one at A and the other at B. A fire was lit at point C.(Solved)
a) Figure 11 shows how an underground room was ventilated. It had two vents, one at A and the other at B. A fire was lit at point C.
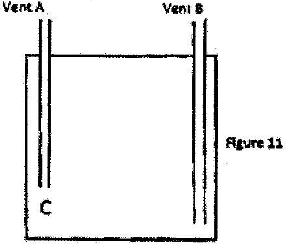
Explain what happened to the ventilation when the fire was lit
b) Explain how a vacuum flask minimizes loss of heat through radiation.
Date posted: April 17, 2017. Answers (1)
- Figure 4 shows identical beakers P and Q full of water at 90oC. Two similar cold wet clothes are wrapped, one around the top of P and the other around the bottom of Q.
(Solved)
Figure 4 shows identical beakers P and Q full of water at 90oC. Two similar cold wet clothes are wrapped, one around the top of P and the other around the bottom of Q.
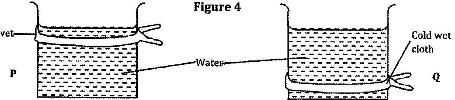
State with a reason the beaker in which the water cools faster.
Date posted: April 17, 2017. Answers (1)
- A paper windmill in a horizontal axis was placed above a candle as shown in Figure 2. When the candle was lit the paper windmill begun to rotate. Explain this observation(Solved)
A paper windmill in a horizontal axis was placed above a candle as shown in Figure 2. When the candle was lit the paper windmill begun to rotate. Explain this observation

Date posted: April 17, 2017. Answers (1)
- Use the following information to answer questions 17 and 18. Two identical empty metal containers P and Q are placed over(Solved)
Use the following information to answer questions a) and b)
Two identical empty metal containers P and Q are placed over identical Bunsen burners and the burners lit. P is dull black while Q is shiny bright. After each container attains a temperature of 100oC the burner are turned off. Identical test tubes containing water are suspended in each container without touching the sides as shown in Figure 3.
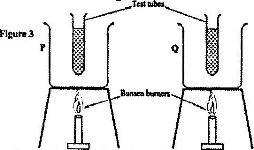
a) Explain why the container Q may become hot faster than P.
b) Explain why the water in test-tube in P becomes hot faster than in Q
Date posted: April 17, 2017. Answers (1)
- Figure 3 shows a hot water bath with metal rods inserted through one of its side. Some wax is fixed at the end of each rod. Use this information to answer questions
(Solved)
Figure 3 shows a hot water bath with metal rods inserted through one of its side. Some wax is fixed at the end of each rod. Use this information to answer questions
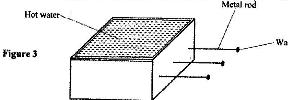
a) What property of metals could be tested using this set-up?
b) Besides the length of the rods that is kept constant, what else should be kept constant when comparing the property for the different metal rods?
Date posted: April 17, 2017. Answers (1)
- The melting point of oxygen is given as -281.3oC. Convert this temperature to Kelvin (K). Figure 4 shows two identical balloons A and B. The balloons were filled with equal amounts(Solved)
The melting point of oxygen is given as -281.3oC. Convert this temperature to Kelvin (K). Figure 4 shows two identical balloons A and B. The balloons were filled with equal amounts

of the same type of gas. The balloons are suspended at distance X1 and X2 from a metal cube filled with boiling water and placed on an insulating material. Use this information to answer question
a) State the mode by which heat travels from the cube to the balloons
b) the face of the cube towards A is bright and shiny and the face towards B is dull black. State with reason the adjustments that should be made on the distances x1 and x2 so that the rate of change of temperature in both balloons is the same
Date posted: April 17, 2017. Answers (1)
- When a Bunsen burner is lit below wire guaze, it is noted that the flame initially burns below the guaze as shown in Figure 5 (i). After sometimes, the flame burns below as well as above the gauze in figure 5 (5) (ii)(Solved)
When a Bunsen burner is lit below wire guaze, it is noted that the flame initially burns below the guaze as shown in Figure 5 (i). After sometimes, the flame burns below as well as above the gauze in figure 5 (5) (ii)
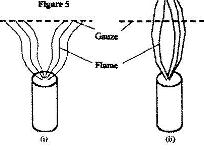
Explain this observation.
Date posted: April 17, 2017. Answers (1)
- Two identical aluminium rods are shown in Figure 4. One rests on metal block and the other on a wooden block. The protruding ends are heated on a Bunsen burner as shown.(Solved)
Two identical aluminium rods are shown in Figure 4. One rests on metal block and the other on a wooden block. The protruding ends are heated on a Bunsen burner as shown.
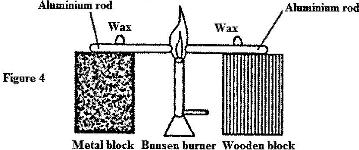
Date posted: April 17, 2017. Answers (1)
- In figure 4 one end of a metal rod is placed in steam and the other end in melting ice. The length of the rod in between in lagged.(Solved)
In figure 4 one end of a metal rod is placed in steam and the other end in melting ice. The length of the rod in between in lagged.
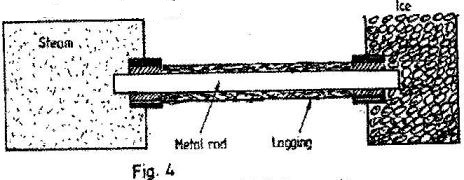
State two factors that determine the rate at which ice melts.
Date posted: April 15, 2017. Answers (1)
- In the set up shown in Fig. 3, water near the top of the boiling tube boils while at the bottom it remains cold.(Solved)
In the set up shown in Fig. 3, water near the top of the boiling tube boils while at the bottom it remains cold.
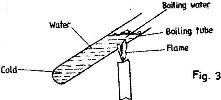
Give a reason for the observation
Date posted: April 15, 2017. Answers (1)
- Figure 2 shows a flat bottomed flask containing some water. It is heated directly with a very hot flame. Explain why the flask is likely to crack(Solved)
Figure 2 shows a flat bottomed flask containing some water. It is heated directly with a very hot flame. Explain why the flask is likely to crack
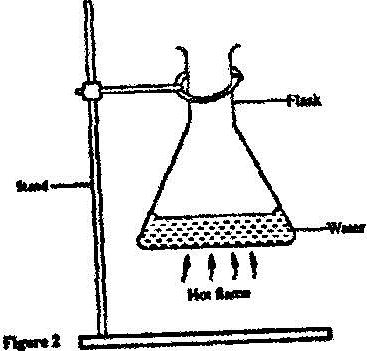
Date posted: April 15, 2017. Answers (1)
- Figure 3 shows an aluminium tube tightly stuck in a steel tube.(Solved)
Figure 3 shows an aluminium tube tightly stuck in a steel tube.

Explain how the two tubes can be separated by applying a temperature change at the same junction given that aluminium expands more than steel for the same temperature rise.
Date posted: April 15, 2017. Answers (1)
- In the set up shown in figure 4, it is observed that the level of the water initially drops before starting to rise.
(Solved)
In the set up shown in figure 4, it is observed that the level of the water initially drops before starting to rise.
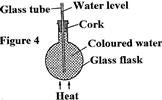
Explain the observation.
Date posted: April 15, 2017. Answers (1)
- Figure 5 shows a flask fitted with a glass tube dipped into a beaker containing water at room temperature. The cork fixing the glass tube to the flask is airtight.(Solved)
Figure 5 shows a flask fitted with a glass tube dipped into a beaker containing water at room temperature. The cork fixing the glass tube to the flask is airtight.
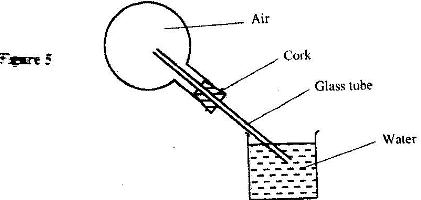
Use the information and the figure to answer the questions
a) State what is observed when ice-cold water is poured on the flask
b) Give a reason for the observation
Date posted: April 13, 2017. Answers (1)
- Figure 4 shows ma bimetallic thermometer
(Solved)
Figure 4 shows ma bimetallic thermometer
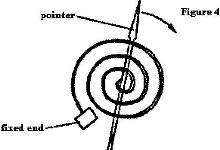
Explain how a rise in temperature causes the pointer to move in the direction shown
Date posted: April 13, 2017. Answers (1)
- Figure 10 shows a fire alarm circuit(Solved)
Figure 10 shows a fire alarm circuit

Explain how the alarm functions
Date posted: April 13, 2017. Answers (1)
- Figure 1 shows a circuit diagram for controlling the temperature of a room(Solved)
Figure 1 shows a circuit diagram for controlling the temperature of a room

i) State and explain the purpose of the bimetallic strip.
ii) Describe how the circuit controls the temperature when the switch S is closed.
Date posted: April 13, 2017. Answers (1)
- Figure 3 shows the arrangement of molecules in the three states of matter(Solved)
Figure 3 shows the arrangement of molecules in the three states of matter
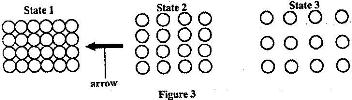
a) Name the process represented by the arrow.
b) State the reason for the arrangement of molecules in state 3.
Date posted: April 13, 2017. Answers (1)
- Figure 4 (i) shows a beaker filled with water. Some potassium permanganate was gently introduced at the bottom of the beaker at the position shown(Solved)
Figure 4 (i) shows a beaker filled with water. Some potassium permanganate was gently introduced at the bottom of the beaker at the position shown
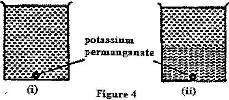
Figure 4 (ii) shows the appearance of the liquid after about 30 minutes. Explain how this appearance was caused
Date posted: April 13, 2017. Answers (1)