a) (i) T = Iron (II) sulphide
ii) U = Hydrogen sulphide
b) When lead (II) acetate paper is inserted in the gas, it turns black
johnmulu answered the question on April 20, 2017 at 07:41
-
A sample of air contaminated with carbon (II) oxide and sulphur (IV) oxide was passed through the apparatus shown in the diagram below.
(Solved)
A sample of air contaminated with carbon (II) oxide and sulphur (IV) oxide was passed through the apparatus shown in the diagram below.
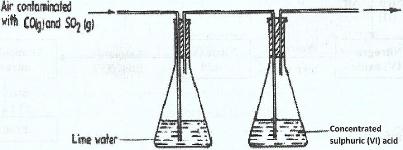
Which contaminant was removed by passing the contaminated air through the apparatus Explain
Date posted:
April 20, 2017
.
Answers (1)
-
The diagram below represents the extraction of sulphur by Frasch process
(Solved)
The diagram below represents the extraction of sulphur by Frasch process
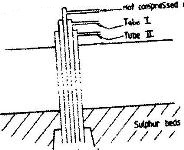
a) Name the substance that passes through tube;
I.
II.
b) What is the purpose of hot compressed air in the process?
Date posted:
April 20, 2017
.
Answers (1)
-
The table below shows the observations made when aqueous ammonia was added to cations of elements E, F and G until in excess.
(Solved)
The table below shows the observations made when aqueous ammonia was added to cations of elements E, F and G until in excess.

i) Select the cation that is likely to be Zn2+
ii) Given that the formula of the cation of element E is E2+, write the ionic equation for the reaction between E2+ (aq) and aqueous ammonia.
Date posted:
April 20, 2017
.
Answers (1)
-
Study the flow chart below and answer the questions that follow
(Solved)
Study the flow chart below and answer the questions that follow

i) Identify gas J.
ii) Using oxidation number, show that ammonia is the reducing agent in step (VI)
iii) Write the equation for the reaction that occurs in step (V)
Date posted:
April 20, 2017
.
Answers (1)
-
Dry ammonia and dry oxygen were reacted as shown in the diagram below
(Solved)
Dry ammonia and dry oxygen were reacted as shown in the diagram below
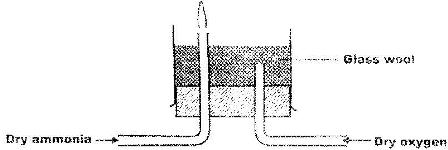
a) what is the purpose of the glass wool?
b) What products would be formed if red hot platinum was introduced into a mixture of ammonia and oxygen
Date posted:
April 20, 2017
.
Answers (1)
-
A student used the set up below to prepare a sample of nitrogen gas
(Solved)
A student used the set up below to prepare a sample of nitrogen gas
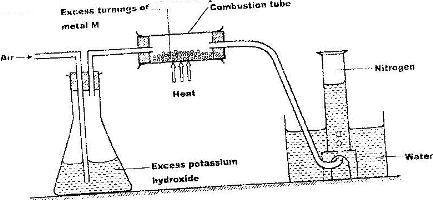
a) State the function of potassium hydroxide in the set up
b) Give a suitable metal M for use in the combustion tube
c) Give a reason why the nitrogen gas obtained is not pure
Date posted:
April 20, 2017
.
Answers (1)
-
Study the set-up below and answer the questions that follow.
(Solved)
Study the set-up below and answer the questions that follow.
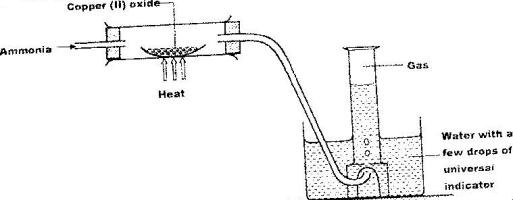
a) Write an equation for the reaction between ammonia and copper (II) oxide
b) During the experiment, the colour of the contents in the water trough changed. State the colour change observed and give an explanation.
Date posted:
April 20, 2017
.
Answers (1)
-
Dry ammonia gas was passed over heated lead (II) oxide and the products passed over anhydrous copper (II) sulphate as shown in the diagram below.
(Solved)
Dry ammonia gas was passed over heated lead (II) oxide and the products passed over anhydrous copper (II) sulphate as shown in the diagram below.
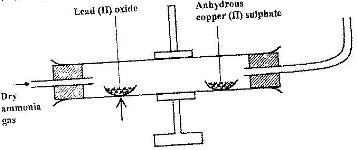
State:
a) Two observations made in the combustion tube
b) The property of ammonia gas in this experiment.
Date posted:
April 20, 2017
.
Answers (1)
-
Figure 1 shows a magnified scale of a micrometer screw gauge
(Solved)
Figure 1 shows a magnified scale of a micrometer screw gauge

Record the reading indicated
Date posted:
April 18, 2017
.
Answers (1)
-
Figure 2 shows a flask filled with water. The flask is fitted with a cork through which a tube is inserted. When the flask is cooled, the water level rises slightly, and then falls steadily.
(Solved)
Figure 2 shows a flask filled with water. The flask is fitted with a cork through which a tube is inserted. When the flask is cooled, the water level rises slightly, and then falls steadily.
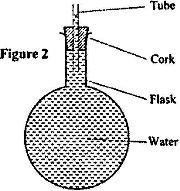
Explain this observation.
Date posted:
April 15, 2017
.
Answers (1)
-
The scheme below shows some reaction sequence starting with solid N. Study it and answer the questions that follow.
(Solved)
The scheme below shows some reaction sequence starting with solid N. Study it and answer the questions that follow.
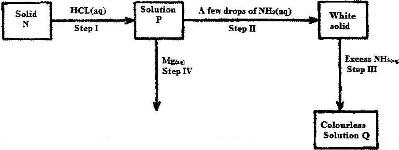
a) write the formula of the complex ions in solution Q.
b) Write an equation for the reaction in step IV.
Date posted:
April 15, 2017
.
Answers (1)
-
In the laboratory, small quantities of nitric (V) acid can be generated using the following set up. Study it and answer the questions that follow
(Solved)
In the laboratory, small quantities of nitric (V) acid can be generated using the following set up. Study it and answer the questions that follow
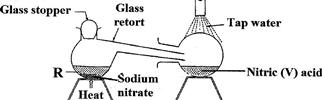
i) Give the name of substance R.
ii) Name one other substance that can be used in place of sodium nitrate.
iii) What is the purpose of using tap water in the set up above?
Date posted:
April 15, 2017
.
Answers (1)
-
Study the flow chart below and answer the questions that follow.
(Solved)
Study the flow chart below and answer the questions that follow.
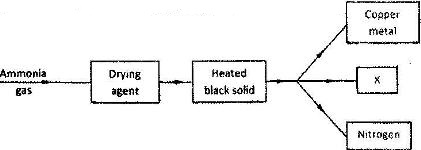
a) name the suitable drying agent for ammonia
b) Describe one chemical test for ammonia
c) Name X.
Date posted:
April 15, 2017
.
Answers (1)
-
The set up below shows how nitrogen gas is prepared in the laboratory.
(Solved)
The set up below shows how nitrogen gas is prepared in the laboratory.

a) describe how nitrogen gas is formed in the flask.
b) Nitrogen is inert. State one use of the gas based on this property.
Date posted:
April 15, 2017
.
Answers (1)
-
When magnesium was burnt in air, a solid mixture was formed. On addition of water to the mixture a gas which turned moist red litmus paper blue was evolved. Explain these observations.
(Solved)
When magnesium was burnt in air, a solid mixture was formed. On addition of water to the mixture a gas which turned moist red litmus paper blue was evolved. Explain these observations.

Date posted:
April 15, 2017
.
Answers (1)
-
The diagram below shows a set-up that was used to prepare and collect a sample of nitric (V) acid.
(Solved)
The diagram below shows a set-up that was used to prepare and collect a sample of nitric (V) acid.

i) Give a reason why it is possible to separate nitric (V) acid from sulphuric (VI) acid in the set-up
ii) Name another substance that can be used instead of potassium nitrate.
iii) Give one use of nitric (V) acid
Date posted:
April 15, 2017
.
Answers (1)
-
The flow chart below shows some reactions starting with lead (II) nitrate. Study it and answer the questions that follow.
(Solved)
The flow chart below shows some reactions starting with lead (II) nitrate. Study it and answer the questions that follow.

i) State the condition necessary in step 1.
ii) Identify:
I. Reagent K.
II. Gas Q.
III. Acidic products S and R.
Date posted:
April 15, 2017
.
Answers (1)
-
Ammonium nitrate was heated as shown in the set-up below.
(Solved)
Ammonium nitrate was heated as shown in the set-up below.
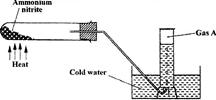
a) Identify gas A
b) State and explain a precaution that must be taken before heating is stopped.
Date posted:
April 15, 2017
.
Answers (1)
-
Ammonia gas was passed into water as shown below
(Solved)
Ammonia gas was passed into water as shown below
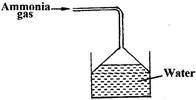
a) Explain why the pH of solution is above 7
b) What is the use of inverted tunnel?
Date posted:
April 15, 2017
.
Answers (1)
-
Ammonium nitrate was gently heated and the products collected as shown in the diagram below.
(Solved)
Ammonium nitrate was gently heated and the products collected as shown in the diagram below.
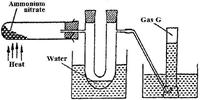
Describe one chemical and one physical method that can be used to identify gas G.
Date posted:
April 15, 2017
.
Answers (1)