i) Aluminium chloride
ii) - Prevent water from entering the apparatus hence preventing hydrolysis of aluminium chloride
-React with excess chlorine to prevent environment pollution
johnmulu answered the question on April 20, 2017 at 14:14
- In an experiment, a test-tube full of chlorine water was inverted in chlorine water as shown in the diagram below and the set up left in sunlight for one day(Solved)
In an experiment, a test-tube full of chlorine water was inverted in chlorine water as shown in the diagram below and the set up left in sunlight for one day
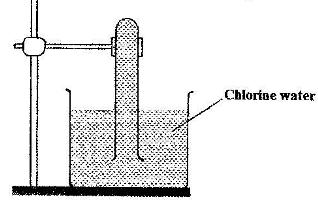
After one day, a gas was found to have collected in the test-tube
a) Identify the gas
b) What will happen to the pH of the solution in the beaker after one day? Give an explanation
Date posted: April 20, 2017. Answers (1)
- The diagram below represents the set-up that was used to prepare and collect hydrogen chloride gas in the laboratory(Solved)
The diagram below represents the set-up that was used to prepare and collect hydrogen chloride gas in the laboratory

a)State the purpose of concentrated sulphuric (VI) acid in the wash bottle.
b) Hydrogen chloride gas is dissolved in water to make hydrochloric acid. State one use of hydrochloric acid.
Date posted: April 20, 2017. Answers (1)
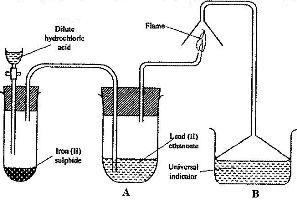
- The set-up below was used to prepare hydrogen chloride gas and react it with iron powder.Study it and answer the questions that follow.(Solved)
The set-up below was used to prepare hydrogen chloride gas and react it with iron powder.Study it and answer the questions that follow.
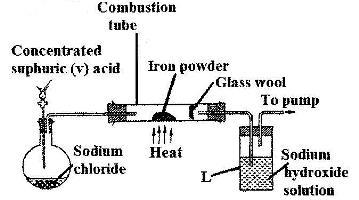
At the end of the reaction, the iron powder turned into a light green solid
a) Identify the light green solid
b) At the beginning of the experiment, the pH of the solution in container L was about 14. At the end, the pH was found to be 2. Explain.
Date posted: April 20, 2017. Answers (1)
- A student set out to prepare iron (III) chlorine using the apparatus shown in the diagram below.(Solved)
A student set out to prepare iron (III) chlorine using the apparatus shown in the diagram below.
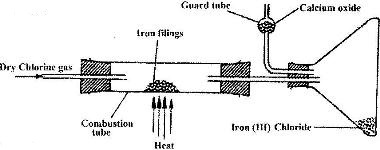
i) Explain why: I. It is necessary to pass chlorine gas through the apparatus before heating begins
II. calcium oxide would be preferred to calcium chloride in the guard tube
ii) What property of iron (III) chloride makes it possible to be collected as shown in the diagram?
Date posted: April 20, 2017. Answers (1)
- The diagram below shows a set up for the laboratory preparation and collection of dry chlorine gas(Solved)
The diagram below shows a set up for the laboratory preparation and collection of dry chlorine gas
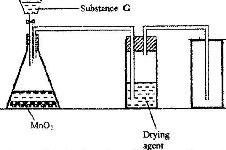
a) Name
i) Substance G
ii) A suitable drying agent
b) What property of chlorine makes it possible for it to be collected as shown in the diagram?
Date posted: April 20, 2017. Answers (1)
- In an experiment, dry hydrogen chloride gas was passed through heated zinc turnings as shown in the diagram below. The gas produced was then passed through heated lead (II) oxide.(Solved)
In an experiment, dry hydrogen chloride gas was passed through heated zinc turnings as shown in the diagram below. The gas produced was then passed through heated lead (II) oxide.
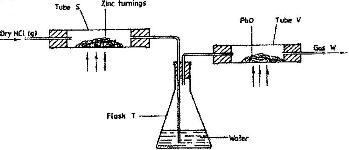
i) What is the function of water in the flask?
ii) How would the total mass of tube V and its contents compare before and after the experiment? Explain.
Date posted: April 20, 2017. Answers (1)
- In an experiment, chlorine gas was passed into moist hydrogen sulphide in a boiling tube as shown in the diagram.(Solved)
In an experiment, chlorine gas was passed into moist hydrogen sulphide in a boiling tube as shown in the diagram.
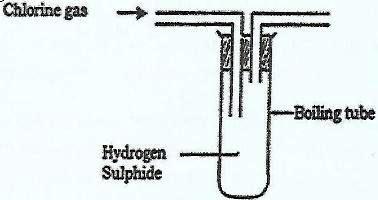
a) What observation was made in the boiling tube?
b) What precaution should be taken in carrying out this experiment? Give a reason.
c) Distinguish between the bleaching action of chlorine and that of sulphur(IV) Oxide.
d) Write an equation of the reaction that took place in the boiling tube
Date posted: April 20, 2017. Answers (1)
- Study the flow chart below and answer the questions that follow.(Solved)
Study the flow chart below and answer the questions that follow.
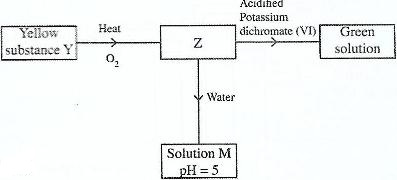
Identify Z and M.
Date posted: April 20, 2017. Answers (1)
- The diagram below shows part of the processes in the manufacture of sulphuric (VI) acid. Study it and answer the question that follow.(Solved)
The diagram below shows part of the processes in the manufacture of sulphuric (VI) acid. Study it and answer the question that follow.
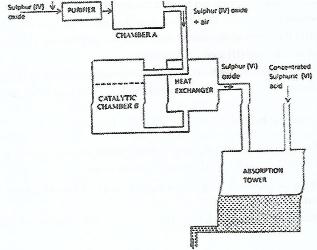
i) Write an equation for the formation of sulphur (IV) oxide from sulphur
ii) What is the role of concentrated sulphuric (VI) acid in chamber A?
iii) Name two catalysts that can be used in the catalystic chamber A?
iv) State two roles of the heat exchanger
Date posted: April 20, 2017. Answers (1)
- The diagram below shows the Frasch process used for extraction of sulphur. Use it to answer the questions that follow.(Solved)
The diagram below shows the Frasch process used for extraction of sulphur. Use it to answer the questions that follow.

i) Identify X.
ii) Why is it necessary to use super heated water in this process?
iii) State two physical properties of sulphur that makes it possible for it to be extracted by this method
Date posted: April 20, 2017. Answers (1)
- The flowchart below shows some of the processes involved in large scale production of sulphuric (IV) acid. Use it to answer the questions that follow.(Solved)
The flowchart below shows some of the processes involved in large scale production of sulphuric (IV) acid. Use it to answer the questions that follow.

a) Describe how oxygen is obtained from air on a large scale.
b) i) Name substance A
ii) Write an equation for the process that takes in the absorption chamber
Date posted: April 20, 2017. Answers (1)
- The set up below was used to prepare a gas and study some of its properties. Study it and answer the questions that follow:
(Solved)
The set up below was used to prepare a gas and study some of its properties. Study it and answer the questions that follow:
aState and explain the observations made in the:
i) Tube labelled A;
ii) State one precaution that should be taken when carrying out this experiment.
Date posted: April 20, 2017. Answers (1)
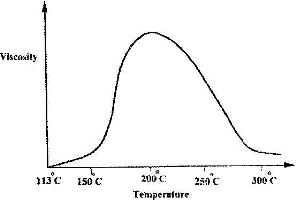
- Below is a sketch of graph showing the change in viscosity (Ease of flow) with temperature when solid sulphur is heated. Describe what happens to the sulphur molecules when is heated from 150oC to about 200oC.(Solved)
Below is a sketch of graph showing the change in viscosity (Ease of flow) with temperature when solid sulphur is heated. Describe what happens to the sulphur molecules when is heated from 150oC to about 200oC.
Date posted: April 20, 2017. Answers (1)
- The diagram below shows some processes that take place during the industrial manufacture of sulphuric (VI) acid(Solved)
The diagram below shows some processes that take place during the industrial manufacture of sulphuric (VI) acid

i) Write the equation for the reaction in which sulphur (VI) oxide gas is produced
ii) Why is it necessary to keep the gases pure and dry?
iii) Describe the process that takes place in chamber G
iv) Name the gases that escape into environment
Date posted: April 20, 2017. Answers (1)
- Study the flow chart below and answer the questions that follow.(Solved)
Study the flow chart below and answer the questions that follow.
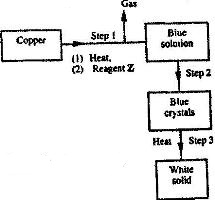
a) Name reagent Z.
b) Describe the process which takes place in step 2
c) Identify the white solid
Date posted: April 20, 2017. Answers (1)
- Dilute hydrochloric acid and solid sodium sulphite were reacted as shown in the set-up below(Solved)
Dilute hydrochloric acid and solid sodium sulphite were reacted as shown in the set-up below
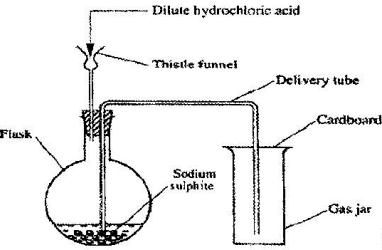
a) Name the gas produced in the flask
b) give two reasons why no gas was collected in the gas jar
Date posted: April 20, 2017. Answers (1)
- The aparatus shown below was set to prepare and collect hydrogen sulphide gas(Solved)
The aparatus shown below was set to prepare and collect hydrogen sulphide gas
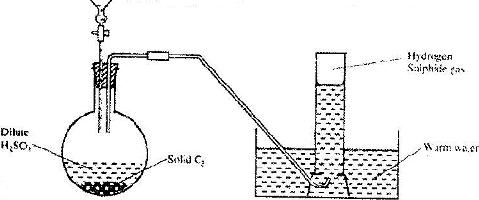
a) Name solid C2
b) Give a reason why warm water is used.
c) What observation would be made if hydrogen sulphide gas was bubbled into a solution of lead (II) nitrate?
Date posted: April 20, 2017. Answers (1)
- Study the flow chart below and answer the questions that follow.(Solved)
Study the flow chart below and answer the questions that follow.
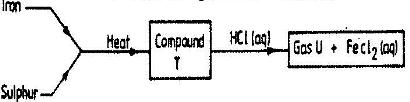
a) Name (i) Compound T
ii) Gas U
b) Give a chemical test that you could use to identify gas U
Date posted: April 20, 2017. Answers (1)
- A sample of air contaminated with carbon (II) oxide and sulphur (IV) oxide was passed through the apparatus shown in the diagram below.(Solved)
A sample of air contaminated with carbon (II) oxide and sulphur (IV) oxide was passed through the apparatus shown in the diagram below.
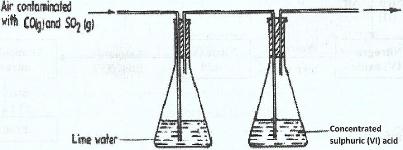
Which contaminant was removed by passing the contaminated air through the apparatus Explain
Date posted: April 20, 2017. Answers (1)
- The diagram below represents the extraction of sulphur by Frasch process(Solved)
The diagram below represents the extraction of sulphur by Frasch process
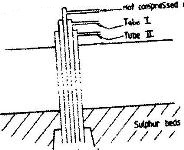
a) Name the substance that passes through tube;
I.
II.
b) What is the purpose of hot compressed air in the process?
Date posted: April 20, 2017. Answers (1)