a) Solution III
b) Solution I and IV: Aluminium oxide is amphoteric hence reacts with both strong acids and strong bases
johnmulu answered the question on April 21, 2017 at 08:45
- The table below shows the solubility of a salt at various temperatures(Solved)
The table below shows the solubility of a salt at various temperatures
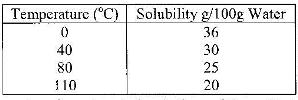
What would happen if a sample of a saturated solution of the salt at 40o C is heated to 80oC.Explain
Date posted: April 21, 2017. Answers (1)
- Study the flow chart below and answer the questions that follow(Solved)
Study the flow chart below and answer the questions that follow

Name:
i) The reagent used in step I
ii) Compound A
Date posted: April 21, 2017. Answers (1)
- The column below was used to soften hard water(Solved)
The column below was used do soften hard water

i) Explain how the hard water was softened as it passed through the column.
ii) After sometimes the material in the column is not able to soften hard water. How can the material be reactivated?
Date posted: April 21, 2017. Answers (1)
- The scheme below shows some reaction sequence starting with solid N.
(Solved)
The scheme below shows some reaction sequence starting with solid N.

a) Identify solid N.
b) Write the formula of the complex ion present in solution Q
Date posted: April 21, 2017. Answers (1)
- Study the information in the table and answer the question below the table.(Solved)
Study the information in the table and answer the question below the table.

Describe how a solid sample of substance A could be obtained from a solid mixture of A and B
Date posted: April 21, 2017. Answers (1)
- Study the scheme below and answer the questions that follow(Solved)
Study the scheme below and answer the questions that follow
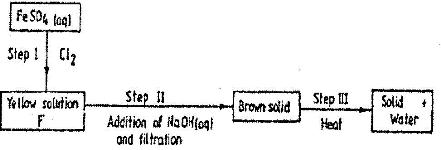
a) Write the formula of the caution present in solution F.
b) What property of chlorine is shown in step I
Date posted: April 21, 2017. Answers (1)
- Study the chart below and answer the questions that follow:
(Solved)
Study the chart below and answer the questions that follow:
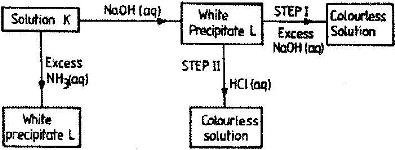
a) Identify: (i) The metal ions in solution K.
ii) The white precipitate L
b) What property of the white precipitate L is illustrated by steps I and II?
Date posted: April 21, 2017. Answers (1)
- The flow charts below show an analysis of a mixture R that contains two salts. Study the analysis and answer the questions that follow.(Solved)
The flow charts below show an analysis of a mixture R that contains two salts. Study the analysis and answer the questions that follow.
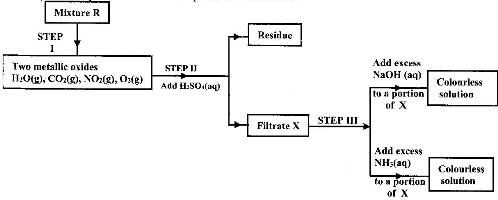
i) What condition is necessary for the process in step I to take place.
ii) Write an ionic equation for the reaction between the cation in filtrate X and aqueous ammonia
iii) What observation would indicate the presence of NO2(g) in step I?
iv) State how water vapour, in step I could be identified.
Date posted: April 21, 2017. Answers (1)
- The table shows how solubility of some substances in water varies with temperature(Solved)
The table shows how solubility of some substances in water varies with temperature
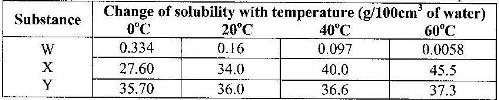
Which of the above substances is likely to be a gas? EXplain
Date posted: April 21, 2017. Answers (1)
- In an experiment, soap solution was added to three separate samples of water. The table below shows the volumes of soap solutions required to form lather with 1000cm3 of each sample of water before and after boiling.(Solved)
In an experiment, soap solution was added to three separate samples of water. The table below shows the volumes of soap solutions required to form lather with 1000cm3 of each sample of water before and after boiling.

a) Which water sample is likely to be soft? Explain
b) Name the cause of change in the volume of soap solution used in sample III
Date posted: April 21, 2017. Answers (1)
- The diagram below represents the set-up that was used to prepare and collect hydrogen chloride gas in the laboratory.(Solved)
The diagram below represents the set-up that was used to prepare and collect hydrogen chloride gas in the laboratory.
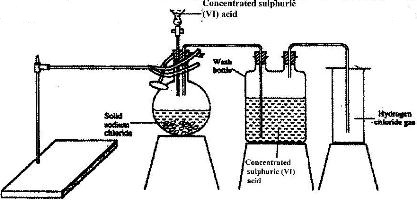
a) State the purpose of concentrated sulphuric (VI) acid in the wash bottle.
b) Write an equation for the reaction between dry hydrogen chloride gas and heated iron
Date posted: April 21, 2017. Answers (1)
- The diagram below is representation of an industrial process for the manufacture of a bleaching powder.(Solved)
The diagram below is representation of an industrial process for the manufacture of a bleaching powder.
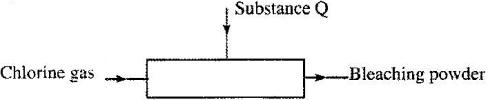
i) Name substance Q.
ii) When the bleaching powder is added to water during washing, a lot of soap is used. Explain.
Date posted: April 21, 2017. Answers (1)
- The set-up below was used to prepare dry hydrogen chlorine gas, and investigate its effect on the heated iron fillings(Solved)
The set-up below was used to prepare dry hydrogen chlorine gas, and investigate its effect on the heated iron fillings
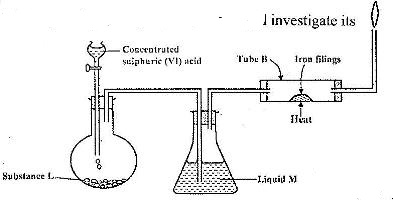
i) Name the substance L.
ii) Name liquid M
iii) What will be observed in tube B?
iv) Why is the gas from tube B burnt?
Date posted: April 21, 2017. Answers (1)
- A gas jar full of chlorine water was inverted over water and allowed to stand for some time.
a) State and explain two observations made in the gas jar after some times.
(Solved)
A gas jar full of chlorine water was inverted over water and allowed to stand for some time.
a) State and explain two observations made in the gas jar after some times.

b) Write the equation for the reaction between chlorine and hot concentrated potassium hydroxide
Date posted: April 21, 2017. Answers (1)
- The diagram below represents a setup for large scale manufacture of hydrochloric acid. Study it and answer the questions that follow(Solved)
The diagram below represents a setup for large scale manufacture of hydrochloric acid. Study it and answer the questions that follow
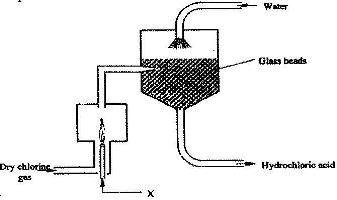
a) Name substance X.
b) What is the purpose of the glass beads?
c) Give two uses of hydrochloric acid
Date posted: April 21, 2017. Answers (1)
- In an experiment, dry chlorine gas was reacted with aluminium as shown in Figure 1.(Solved)
In an experiment, dry chlorine gas was reacted with aluminium as shown in Figure 1.
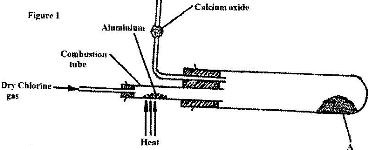
i) Name substance A.
ii) Give two reasons why calcium oxide is used in the set up.
Date posted: April 20, 2017. Answers (1)
- In an experiment, a test-tube full of chlorine water was inverted in chlorine water as shown in the diagram below and the set up left in sunlight for one day(Solved)
In an experiment, a test-tube full of chlorine water was inverted in chlorine water as shown in the diagram below and the set up left in sunlight for one day
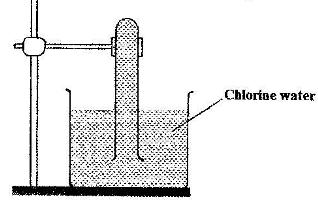
After one day, a gas was found to have collected in the test-tube
a) Identify the gas
b) What will happen to the pH of the solution in the beaker after one day? Give an explanation
Date posted: April 20, 2017. Answers (1)
- The diagram below represents the set-up that was used to prepare and collect hydrogen chloride gas in the laboratory(Solved)
The diagram below represents the set-up that was used to prepare and collect hydrogen chloride gas in the laboratory

a)State the purpose of concentrated sulphuric (VI) acid in the wash bottle.
b) Hydrogen chloride gas is dissolved in water to make hydrochloric acid. State one use of hydrochloric acid.
Date posted: April 20, 2017. Answers (1)
- The set-up below was used to prepare hydrogen chloride gas and react it with iron powder.Study it and answer the questions that follow.(Solved)
The set-up below was used to prepare hydrogen chloride gas and react it with iron powder.Study it and answer the questions that follow.
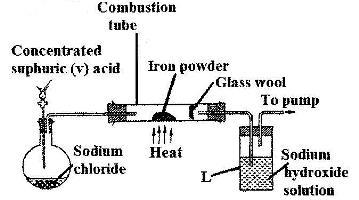
At the end of the reaction, the iron powder turned into a light green solid
a) Identify the light green solid
b) At the beginning of the experiment, the pH of the solution in container L was about 14. At the end, the pH was found to be 2. Explain.
Date posted: April 20, 2017. Answers (1)
- A student set out to prepare iron (III) chlorine using the apparatus shown in the diagram below.(Solved)
A student set out to prepare iron (III) chlorine using the apparatus shown in the diagram below.
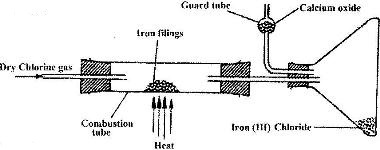
i) Explain why: I. It is necessary to pass chlorine gas through the apparatus before heating begins
II. calcium oxide would be preferred to calcium chloride in the guard tube
ii) What property of iron (III) chloride makes it possible to be collected as shown in the diagram?
Date posted: April 20, 2017. Answers (1)