Hydrochloric acid ionizes fully in solution giving many ions hence has higher electrical conductivity unlike ethanoic acid which ionizes partially giving fewer ions
johnmulu answered the question on April 21, 2017 at 13:25
- Use the flow chart below to answer the questions that follow.
(Solved)
Use the flow chart below to answer the questions that follow.
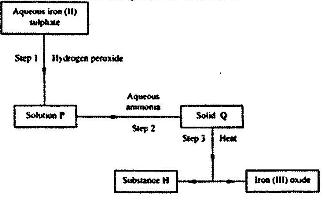
a) What observation would be made in step I?
b) Name another substance that could be used in step 2.
c) Excess aqueous ammonia is added to copper (II) hydroxide.
Date posted: April 21, 2017. Answers (1)
- The solubility curve of potassium nitrate is shown below(Solved)
The solubility curve of potassium nitrate is shown below

a) Determine the solubility of potassium nitrate at 50oC
b) determine the molar concentration of saturated potassium nitrate at 50o(K = 39.0; 0 = 16.0; N = 14.0 and density of water 1g/cm3)
Date posted: April 21, 2017. Answers (1)
- The table below gives the solubilities of substances J, K and L at different temperatures(Solved)
The table below gives the solubilities of substances J, K and L at different temperatures

select the substance which, when dissolved in water, heat is given out. Give a reason.
Date posted: April 21, 2017. Answers (1)
- The graph shows how the pH value of soil in a farm changed over a period of time(Solved)
The graph shows how the pH value of soil in a farm changed over a period of time
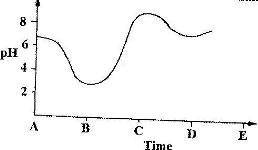
i) Describe how the pH of the soil can be determined
ii) State one factor that may have been responsible for the change in the soil pH in the time interval AB.
Date posted: April 21, 2017. Answers (1)
- Equal volumes of 1 M monobasic acids L and M were each reacted with excess magnesium turnings. The table below shows the volumes of the produced after one minute.
(Solved)
Equal volumes of 1 M monobasic acids L and M were each reacted with excess magnesium turnings. The table below shows the volumes of the produced after one minute.

Explain the differences in the volumes of the gas produced
Date posted: April 21, 2017. Answers (1)
- The set-up below was used to demonstrate the effect of heat on hard water(Solved)
The set-up below was used to demonstrate the effect of heat on hard water
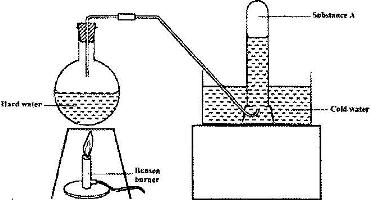
a) Name substance A.
b) Explain why the heating of hard water produced substance A.
Date posted: April 21, 2017. Answers (1)
- In an experiment, student put equal volumes of mixtures of ethanoic acid in water and ethanoic acid in hexane in two test-tubes as shown below. In each test-tube, equal amounts of solid hydrogen carbonate were added.(Solved)
In an experiment, student put equal volumes of mixtures of ethanoic acid in water and ethanoic acid in hexane in two test-tubes as shown below. In each test-tube, equal amounts of solid hydrogen carbonate were added.
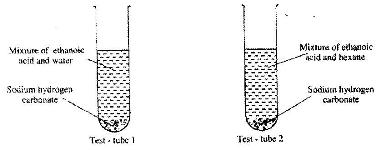
a) State the observation which was made in each test-tube.
b) Explain the observation in (a) above.
Date posted: April 21, 2017. Answers (1)
- The table below shows the tests carried out on a sample of water and the results obtained.
(Solved)
The table below shows the tests carried out on a sample of water and the results obtained.

a) Identify the anion present in the water.
b) Write the formula of the complex ion formed in II
Date posted: April 21, 2017. Answers (1)
- In an experiment equal amounts of magnesium powder were placed into test-tube 1 and 2 as shown below(Solved)
In an experiment equal amounts of magnesium powder were placed into test-tube 1 and 2 as shown below
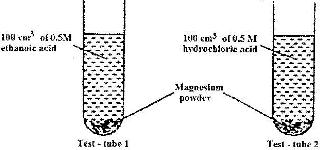
Explain why the amount of hydrogen gas liberated in test - tube 2 is greater than in test-tube 1 before the reaction is complete
Date posted: April 21, 2017. Answers (1)
- Study the information in the table below and answer the questions that follow. (The letters do not represent the actual symbols of the element)(Solved)
Study the information in the table below and answer the questions that follow. (The letters do not represent the actual symbols of the element)

i) What is the general name to the group in which elements P, Q and R belong?
ii) What is meant by ionization energy?
iii) Explain why element P have the highest ionization energy.
Date posted: April 21, 2017. Answers (1)
- The flow chart below shows the various stages of water treatment. Study it and answer the questions that follow.(Solved)
The flow chart below shows the various stages of water treatment. Study it and answer the questions that follow.

i) Which substance are likely to be removed in filtration unit I?
ii) What is the name of the process Y?
iii) What is the purpose of:
I. Process Y
II. Addition of sodium hypochlorite
Date posted: April 21, 2017. Answers (1)
- The table below shows the tests carried out on separate samples of water drawn from a well and the results obtained.(Solved)
The table below shows the tests carried out on separate samples of water drawn from a well and the results obtained.

a) Identify the cation and the anion present in the water.
b) Write an ionic equation for the reaction which takes place in test (iii).
Date posted: April 21, 2017. Answers (1)
- Study the solubility curves below and answer the question that follows.(Solved)
Study the solubility curves below and answer the question that follows.
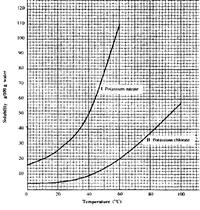
What happens when a solution containing 40 g of potassium chlorate and 40 g of potassium nitrate in 100 g of water at 90oC is cooled 40oC? Explain.
Date posted: April 21, 2017. Answers (1)
- The table below shows the pH values of solutions I, II, and IV.(Solved)
The table below shows the pH values of solutions I, II, and IV.

a) Which solution is likely to be that of calcium hydroxide?
b) Select the solution in which a sample of aluminium oxide is likely to dissolve. Give a reason for your answer
Date posted: April 21, 2017. Answers (1)
- The table below shows the solubility of a salt at various temperatures(Solved)
The table below shows the solubility of a salt at various temperatures
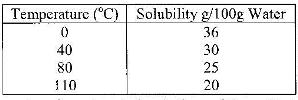
What would happen if a sample of a saturated solution of the salt at 40o C is heated to 80oC.Explain
Date posted: April 21, 2017. Answers (1)
- Study the flow chart below and answer the questions that follow(Solved)
Study the flow chart below and answer the questions that follow

Name:
i) The reagent used in step I
ii) Compound A
Date posted: April 21, 2017. Answers (1)
- The column below was used to soften hard water(Solved)
The column below was used do soften hard water

i) Explain how the hard water was softened as it passed through the column.
ii) After sometimes the material in the column is not able to soften hard water. How can the material be reactivated?
Date posted: April 21, 2017. Answers (1)
- The scheme below shows some reaction sequence starting with solid N.
(Solved)
The scheme below shows some reaction sequence starting with solid N.

a) Identify solid N.
b) Write the formula of the complex ion present in solution Q
Date posted: April 21, 2017. Answers (1)
- Study the information in the table and answer the question below the table.(Solved)
Study the information in the table and answer the question below the table.

Describe how a solid sample of substance A could be obtained from a solid mixture of A and B
Date posted: April 21, 2017. Answers (1)
- Study the scheme below and answer the questions that follow(Solved)
Study the scheme below and answer the questions that follow
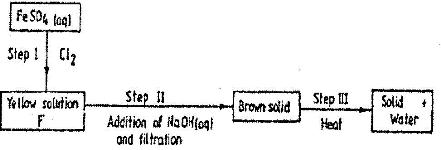
a) Write the formula of the caution present in solution F.
b) What property of chlorine is shown in step I
Date posted: April 21, 2017. Answers (1)