i) I. Van der waals forces and hydrogen bonding
II. Van des waals forces
ii) I. The separation distance is smaller during fusion than during vaporization hence requires much lower
energy than in vaporization and vice versa
II. Heating time NP is far much less than heating time in QR
johnmulu answered the question on April 27, 2017 at 06:57
- In the flow chart below, some processes have been identified and others labeled. Study it and answer the questions that follow.(Solved)
In the flow chart below, some processes have been identified and others labeled. Study it and answer the questions that follow.
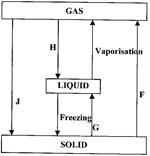
i) Give the names of the processes:
I. H
II. G
ii) Name one substance that can undergo process F when left in an open container in the laboratory.
iii) The process J is called deposition. Using water as an example, write an equation that represents the process of deposition.
Date posted: April 27, 2017. Answers (1)
- Two different samples of water (I and II) were tested with soap solution(Solved)
Two different samples of water (I and II) were tested with soap solution. Sample II was further subjected to two other processes before adding soap. 20 cm3 of each sample of water was shaken with soap solution in a boiling tube until a permanent lather was obtained. The results are as shown in the table below.
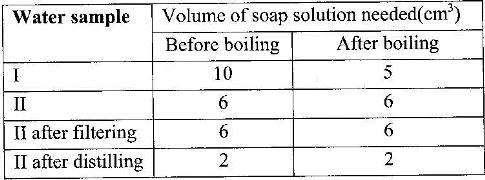
i) Identify the water sample that had temporary hardness. Explain your answer
ii) Explain why the results for sample II are different after distilling but remain unchanged after filtering
iii) State two disadvantages of using both water samples for domestic purposes.
Date posted: April 27, 2017. Answers (1)
- The table below shows the pH values of solutions A, B, C and D.(Solved)
The table below shows the pH values of solutions A, B, C and D.

Select solutions in which a sample of lead (II) hydroxide is likely to dissolve. Give reasons for each solution selected.
Date posted: April 27, 2017. Answers (1)
- The table below gives the solubilities of substances T and U at 10o C and 40oC(Solved)
The table below gives the solubilities of substances T and U at 10o C and 40oC

When an aqueous mixture containing 55 g of T and 12 g of U at 80o C was cooled to 10oC, crystals formed.
a) Identify the crystals formed.
b) determine the mass of the crystals formed
c) Name the method used to obtain the crystals
Date posted: April 27, 2017. Answers (1)
- Study the information in the table below and answer the questions that follow.(Solved)
Study the information in the table below and answer the questions that follow.
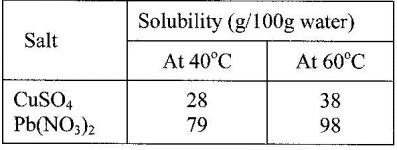
A mixture containing 35g of CuSO4 and 78 g of Pb(NO3)2 in 100 g of water at 60o was cooled to 40oC
a) What salt crystallized out? Give a reason.
b) Calculate the mass of salt that crystallised out.
Date posted: April 21, 2017. Answers (1)
- The curves below show how the electrical conductivity of hydrochloric and ethanoic acids vary with concentration.(Solved)
The curves below show how the electrical conductivity of hydrochloric and ethanoic acids vary with concentration.
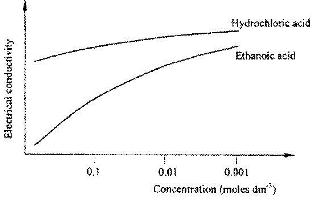
Explain why the electrical conductivity of 0.01 M hydrochloric acid is higher than that of 0.01 M ethanoic acid
Date posted: April 21, 2017. Answers (1)
- Use the flow chart below to answer the questions that follow.
(Solved)
Use the flow chart below to answer the questions that follow.
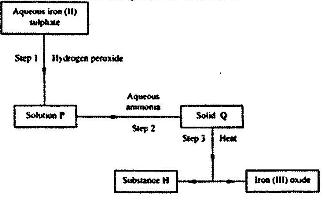
a) What observation would be made in step I?
b) Name another substance that could be used in step 2.
c) Excess aqueous ammonia is added to copper (II) hydroxide.
Date posted: April 21, 2017. Answers (1)
- The solubility curve of potassium nitrate is shown below(Solved)
The solubility curve of potassium nitrate is shown below

a) Determine the solubility of potassium nitrate at 50oC
b) determine the molar concentration of saturated potassium nitrate at 50o(K = 39.0; 0 = 16.0; N = 14.0 and density of water 1g/cm3)
Date posted: April 21, 2017. Answers (1)
- The table below gives the solubilities of substances J, K and L at different temperatures(Solved)
The table below gives the solubilities of substances J, K and L at different temperatures

select the substance which, when dissolved in water, heat is given out. Give a reason.
Date posted: April 21, 2017. Answers (1)
- The graph shows how the pH value of soil in a farm changed over a period of time(Solved)
The graph shows how the pH value of soil in a farm changed over a period of time
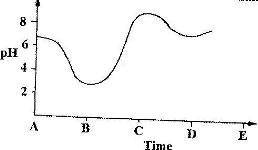
i) Describe how the pH of the soil can be determined
ii) State one factor that may have been responsible for the change in the soil pH in the time interval AB.
Date posted: April 21, 2017. Answers (1)
- Equal volumes of 1 M monobasic acids L and M were each reacted with excess magnesium turnings. The table below shows the volumes of the produced after one minute.
(Solved)
Equal volumes of 1 M monobasic acids L and M were each reacted with excess magnesium turnings. The table below shows the volumes of the produced after one minute.

Explain the differences in the volumes of the gas produced
Date posted: April 21, 2017. Answers (1)
- The set-up below was used to demonstrate the effect of heat on hard water(Solved)
The set-up below was used to demonstrate the effect of heat on hard water
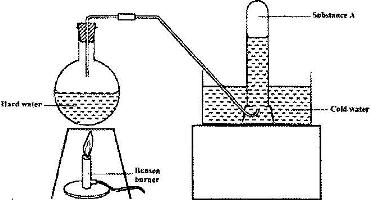
a) Name substance A.
b) Explain why the heating of hard water produced substance A.
Date posted: April 21, 2017. Answers (1)
- In an experiment, student put equal volumes of mixtures of ethanoic acid in water and ethanoic acid in hexane in two test-tubes as shown below. In each test-tube, equal amounts of solid hydrogen carbonate were added.(Solved)
In an experiment, student put equal volumes of mixtures of ethanoic acid in water and ethanoic acid in hexane in two test-tubes as shown below. In each test-tube, equal amounts of solid hydrogen carbonate were added.
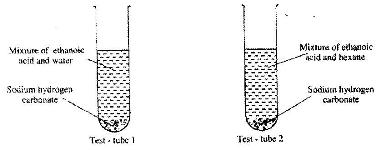
a) State the observation which was made in each test-tube.
b) Explain the observation in (a) above.
Date posted: April 21, 2017. Answers (1)
- The table below shows the tests carried out on a sample of water and the results obtained.
(Solved)
The table below shows the tests carried out on a sample of water and the results obtained.

a) Identify the anion present in the water.
b) Write the formula of the complex ion formed in II
Date posted: April 21, 2017. Answers (1)
- In an experiment equal amounts of magnesium powder were placed into test-tube 1 and 2 as shown below(Solved)
In an experiment equal amounts of magnesium powder were placed into test-tube 1 and 2 as shown below
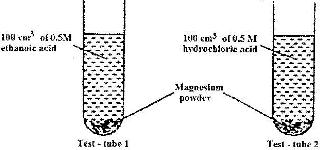
Explain why the amount of hydrogen gas liberated in test - tube 2 is greater than in test-tube 1 before the reaction is complete
Date posted: April 21, 2017. Answers (1)
- Study the information in the table below and answer the questions that follow. (The letters do not represent the actual symbols of the element)(Solved)
Study the information in the table below and answer the questions that follow. (The letters do not represent the actual symbols of the element)

i) What is the general name to the group in which elements P, Q and R belong?
ii) What is meant by ionization energy?
iii) Explain why element P have the highest ionization energy.
Date posted: April 21, 2017. Answers (1)
- The flow chart below shows the various stages of water treatment. Study it and answer the questions that follow.(Solved)
The flow chart below shows the various stages of water treatment. Study it and answer the questions that follow.

i) Which substance are likely to be removed in filtration unit I?
ii) What is the name of the process Y?
iii) What is the purpose of:
I. Process Y
II. Addition of sodium hypochlorite
Date posted: April 21, 2017. Answers (1)
- The table below shows the tests carried out on separate samples of water drawn from a well and the results obtained.(Solved)
The table below shows the tests carried out on separate samples of water drawn from a well and the results obtained.

a) Identify the cation and the anion present in the water.
b) Write an ionic equation for the reaction which takes place in test (iii).
Date posted: April 21, 2017. Answers (1)
- Study the solubility curves below and answer the question that follows.(Solved)
Study the solubility curves below and answer the question that follows.
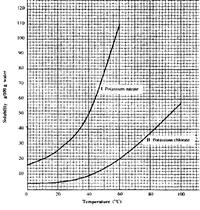
What happens when a solution containing 40 g of potassium chlorate and 40 g of potassium nitrate in 100 g of water at 90oC is cooled 40oC? Explain.
Date posted: April 21, 2017. Answers (1)
- The table below shows the pH values of solutions I, II, and IV.(Solved)
The table below shows the pH values of solutions I, II, and IV.

a) Which solution is likely to be that of calcium hydroxide?
b) Select the solution in which a sample of aluminium oxide is likely to dissolve. Give a reason for your answer
Date posted: April 21, 2017. Answers (1)