i) the blue-green colour of the solution fades since copper ions are removed from the solution
ii) J: Negative charged ions (Cl- and OH-) are attracted to J hence it is the
johnmulu answered the question on April 27, 2017 at 08:03
- The table below gives standard electrode potentials for the metals represented by the letters D, E, F and G. Study it and answer the questions that follow.(Solved)
The table below gives standard electrode potentials for the metals represented by the letters D, E, F and G. Study it and answer the questions that follow.
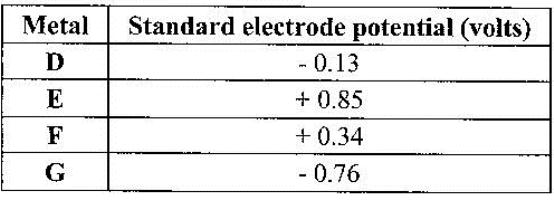
Which metal can be displaced from a solution of its salt by all the other metals in the table? Give a reason.
Date posted: April 27, 2017. Answers (1)
- Study the flow chart below and answer the questions that follow:(Solved)
Study the flow chart below and answer the questions that follow:
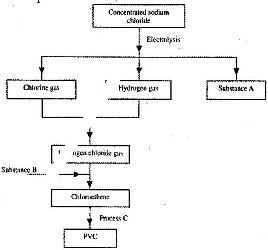
a) Identify substance:
i) A
ii) B
b) Name process C.
c) Give one use of PVC.
Date posted: April 27, 2017. Answers (1)
- The set-up below was used to electroplate a metallic spoon. Study it and answer the questions that follow.(Solved)
The set-up below was used to electroplate a metallic spoon. Study it and answer the questions that follow.
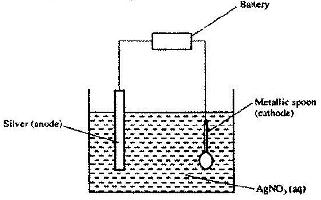
a) Write an ionic equation for the reaction that occurred at the cathode
b) State and explain what happened to the anode.
Date posted: April 27, 2017. Answers (1)
- 100 cm3 of 2 M sulphuric (VI) acid was electrolyzed using the set-up represented by the diagram below(Solved)
100 cm3 of 2 M sulphuric (VI) acid was electrolyzed using the set-up represented by the diagram below
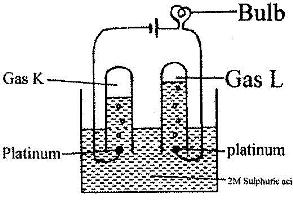
i) Write an equation for the reaction that produces gas L.
ii) Describe how gas K can be identified
iii) Explain the difference in:
I. The volume of the gases produced at the electrodes.
II. Brightness of the bulb if 100 cm3 of 2 M ethanoic acid was used in place of sulphuric (VI) acid.
Date posted: April 27, 2017. Answers (1)
- The diagram below represents a mercury cell that can be used in the industrial manufacture of sodium hydroxide. Study it and answer the questions that follow.(Solved)
The diagram below represents a mercury cell that can be used in the industrial manufacture of sodium hydroxide. Study it and answer the questions that follow.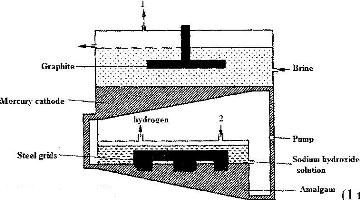
i) Name
I. The raw material introduced at 2
II. another substance that can be used in the cell instead of graphite.
ii) Identify the by – product that comes out at 1
iii) Give
I. One use of sodium hydroxide
II. Two reasons why mercury is recycled.
Date posted: April 27, 2017. Answers (1)
- The set-up below was used during the electrolysis of aqueous magnesium sulphate using inert electrodes.(Solved)
The set-up below was used during the electrolysis of aqueous magnesium sulphate using inert electrodes.

a) Name a suitable pair of electrodes for this experiment.
b) Identify the anions and cations in the solution.
c) Explain the change that occurred to the concentration of magnesium sulphate solution during the experiment
Date posted: April 27, 2017. Answers (1)
- The set –up below was used to electrolyse aqueous copper (II) sulphate.(Solved)
The set –up below was used to electrolyse aqueous copper (II) sulphate.
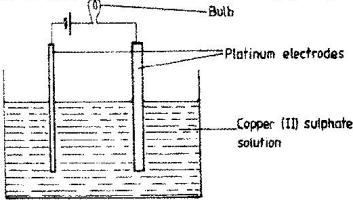
a) Explain why the bulb light brightly at the beginning of the experiment and becomes dim after sometimes.
b) Write the ionic equation of the reaction that took place at the cathode.
Date posted: April 27, 2017. Answers (1)
- Study the diagram below and answer the questions that follow.(Solved)
Study the diagram below and answer the questions that follow.
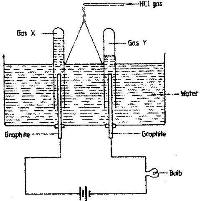
When some hydrogen chloride gas is allowed into water and the mixture stirred, the bulb lights and gases X and Y are formed.
i) Name: gas X and gas Y
ii) Explain why the bulbs does not light before the hydrogen chloride gas is let into water
Date posted: April 27, 2017. Answers (1)
- A solution of hydrogen was allowed to decompose and the oxygen gas given off collected(Solved)
A solution of hydrogen was allowed to decompose and the oxygen gas given off collected. After 5 minutes, substance G was added to the solution of hydrogen peroxide. The total volume of oxygen evolved was plotted against time as shown in the graph below
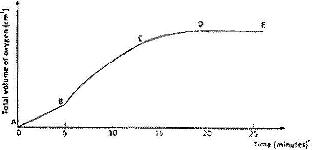
i) Describe the procedure of determining the rate of the reaction at minute 12.
ii) How does the production of oxygen in region AB compare with that in region BC? Explain.
Date posted: April 27, 2017. Answers (1)
- The curve below shows the variation of time against temperature for the reaction between sodium thiosulphate and hydrochloric acid(Solved)
The curve below shows the variation of time against temperature for the reaction between sodium thiosulphate and hydrochloric acid
Explain the shape of the curve.
Date posted: April 27, 2017. Answers (1)
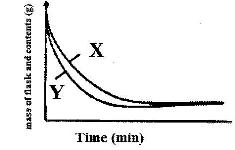
- The curves below represents the change in mass when equal masses of powdered zinc and zinc granules were reacted with excess 2 M hydrochloric acid. Study them and answer the question below(Solved)
The curves below represents the change in mass when equal masses of powdered zinc and zinc granules were reacted with excess 2 M hydrochloric acid. Study them and answer the question below

Which curve represents the reaction with zinc granules? Explain answer.
Date posted: April 27, 2017. Answers (1)
- Ammonia gas is used to manufacture nitric (V) acid as shown below.(Solved)
Ammonia gas is used to manufacture nitric (V) acid as shown below.

i) This process requires the use of a catalyst. In which unit is the catalyst used?
ii) Identify compound A and B.
Date posted: April 27, 2017. Answers (1)
- A student set up the apparatus as shown in the diagram below to prepare and collect dry
Ammonia gas
(Solved)
A student set up the apparatus as shown in the diagram below to prepare and collect dry
Ammonia gas

i) Identify two mistakes in the set up and give a reason for each mistake.
ii) Name a suitable drying agent for ammonia.
iii) Describe one chemical test for ammonia gas
Date posted: April 27, 2017. Answers (1)
- Study the energy level diagram below and answer the question that follows(Solved)
Study the energy level diagram below and answer the question that follows
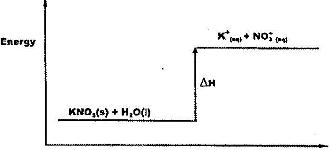
What type of reaction is represented by the diagram?
Date posted: April 27, 2017. Answers (1)
- A student investigated a property of acids M and N by reacting equal volumes of acid M and N of the same concentration with equal volumes of 2 M potassium hydroxide. The results were recorded in the table below(Solved)
A student investigated a property of acids M and N by reacting equal volumes of acid M and N of the same concentration with equal volumes of 2 M potassium hydroxide. The results were recorded in the table below

a) Which of the acids is likely to be a weak acid? Explain.
b) Write the equation for the reaction between ethanoic acid and potassium hydroxide.
Date posted: April 27, 2017. Answers (1)
- 50 cm3 of 1 M copper (II) sulphate solution was placed in 100 cm(Solved)
50 cm3 of 1 M copper (II) sulphate solution was placed in 100 cm 3 plastic beaker. The temperature of the solution was measured. Excess metal A powder was added to the solution, the mixture stirred, and the maximum temperature of the mixture measured. The experiment was repeated using powders of metals B and C. The results obtained are given in the table below;

i) Arrange the metals A, B, C and copper in order of reactivity starting with the least reactive. Give reasons for the order.
ii) Other than temperature change, state one other observation that was made when the most reactive metal was added to copper (II) sulphate solution
Date posted: April 27, 2017. Answers (1)
- Figure 4 shows the heating curve for water.(Solved)
Figure 4 shows the heating curve for water.
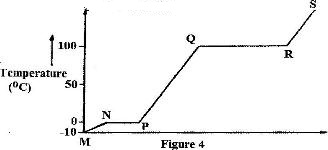
i) Give the names of the intermolecular forces of attraction in the segments:
I. MN
II. RS
ii) The heats of fusion and vaporization of water are 334.4 Jg-1 and 1159.4 g-1 respectively
I. Explain why there is a big difference between the two.
II. How is the difference reflected in the curve?
Date posted: April 27, 2017. Answers (1)
- In the flow chart below, some processes have been identified and others labeled. Study it and answer the questions that follow.(Solved)
In the flow chart below, some processes have been identified and others labeled. Study it and answer the questions that follow.
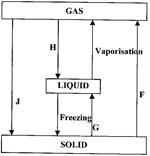
i) Give the names of the processes:
I. H
II. G
ii) Name one substance that can undergo process F when left in an open container in the laboratory.
iii) The process J is called deposition. Using water as an example, write an equation that represents the process of deposition.
Date posted: April 27, 2017. Answers (1)
- Two different samples of water (I and II) were tested with soap solution(Solved)
Two different samples of water (I and II) were tested with soap solution. Sample II was further subjected to two other processes before adding soap. 20 cm3 of each sample of water was shaken with soap solution in a boiling tube until a permanent lather was obtained. The results are as shown in the table below.
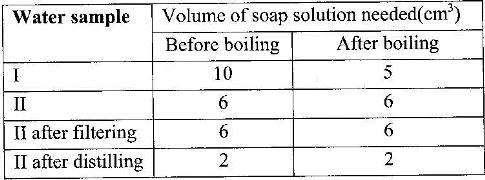
i) Identify the water sample that had temporary hardness. Explain your answer
ii) Explain why the results for sample II are different after distilling but remain unchanged after filtering
iii) State two disadvantages of using both water samples for domestic purposes.
Date posted: April 27, 2017. Answers (1)
- The table below shows the pH values of solutions A, B, C and D.(Solved)
The table below shows the pH values of solutions A, B, C and D.

Select solutions in which a sample of lead (II) hydroxide is likely to dissolve. Give reasons for each solution selected.
Date posted: April 27, 2017. Answers (1)