a) I. $D^{2+}_{(l)} + 2e^- \rightarrow D_{(s)}$
II. $2Br^-(l) \rightarrow Br^-(l) \rightarrow Br_2(g) + 2e^-$
b) Carbon : It will not be attacked by bromine gas and D reacts with bromine vapour
c) Bromine gas is poisonous or toxic
johnmulu answered the question on May 15, 2017 at 08:51
-
The diagram below represents an experiment that was set up to investigate movement of ions during electrolysis.
(Solved)
The diagram below represents an experiment that was set up to investigate movement of ions during electrolysis.

When the circuit was completed, it was noticed that a blue colour spread towards the right.
a) Explain this observation.
b) Write the equation for the reaction that occurred at the anode.
Date posted:
May 15, 2017
.
Answers (1)
-
The diagram below shows the apparatus that can be used to electrolyze acidified water obtain hydrogen and oxygen gases. Study it and answer the questions that follow.
(Solved)
The diagram below shows the apparatus that can be used to electrolyze acidified water obtain hydrogen and oxygen gases. Study it and answer the questions that follow.

i) Identify the electrode at which oxidation takes place.
ii) Give a reason why it is necessary to acidify the water.
iii) Explain why hydrochloric acid is not used to acidify the water.
Date posted:
May 15, 2017
.
Answers (1)
-
The set-up below was used to electrolyse molten lead (II) iodine
(Solved)
The set-up below was used to electrolyse molten lead (II) iodine

i) State the observation that was made at the anode during the electrolysis. Give a reason for your answer.
ii) A current of 0.5 A was passed for two hours. Calculate the mass of lead that was deposited (pb = 2071F = 9, 500C)
Date posted:
April 27, 2017
.
Answers (1)
-
An electric current was passed through a concentrated solution of copper (II) chloride as shown in the diagram below.
(Solved)
An electric current was passed through a concentrated solution of copper (II) chloride as shown in the diagram below.

i) Explain the observation that would be made on the electrolyte as the experiment progresses
ii) Which of the electrodes is the anode? Explain.
Date posted:
April 27, 2017
.
Answers (1)
-
The set-up below was used to electroplate a metallic spoon. Study it and answer the questions that follow.
(Solved)
The set-up below was used to electroplate a metallic spoon. Study it and answer the questions that follow.
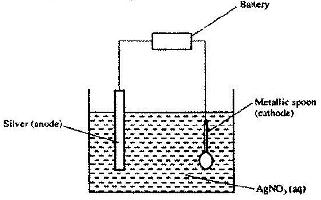
a) Write an ionic equation for the reaction that occurred at the cathode
b) State and explain what happened to the anode.
Date posted:
April 27, 2017
.
Answers (1)
-
100 cm3 of 2 M sulphuric (VI) acid was electrolyzed using the set-up represented by the diagram below
(Solved)
100 cm3 of 2 M sulphuric (VI) acid was electrolyzed using the set-up represented by the diagram below
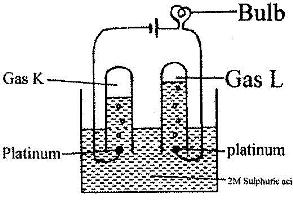
i) Write an equation for the reaction that produces gas L.
ii) Describe how gas K can be identified
iii) Explain the difference in:
I. The volume of the gases produced at the electrodes.
II. Brightness of the bulb if 100 cm3 of 2 M ethanoic acid was used in place of sulphuric (VI) acid.
Date posted:
April 27, 2017
.
Answers (1)
-
The diagram below represents a mercury cell that can be used in the industrial manufacture of sodium hydroxide. Study it and answer the questions that follow.
(Solved)
The diagram below represents a mercury cell that can be used in the industrial manufacture of sodium hydroxide. Study it and answer the questions that follow.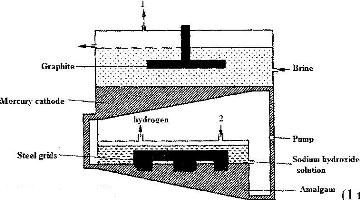
i) Name
I. The raw material introduced at 2
II. another substance that can be used in the cell instead of graphite.
ii) Identify the by – product that comes out at 1
iii) Give
I. One use of sodium hydroxide
II. Two reasons why mercury is recycled.
Date posted:
April 27, 2017
.
Answers (1)
-
The set-up below was used during the electrolysis of aqueous magnesium sulphate using inert electrodes.
(Solved)
The set-up below was used during the electrolysis of aqueous magnesium sulphate using inert electrodes.

a) Name a suitable pair of electrodes for this experiment.
b) Identify the anions and cations in the solution.
c) Explain the change that occurred to the concentration of magnesium sulphate solution during the experiment
Date posted:
April 27, 2017
.
Answers (1)
-
The set –up below was used to electrolyse aqueous copper (II) sulphate.
(Solved)
The set –up below was used to electrolyse aqueous copper (II) sulphate.
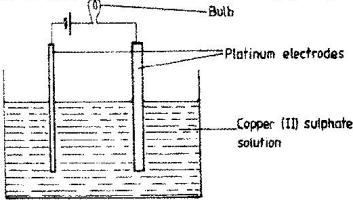
a) Explain why the bulb light brightly at the beginning of the experiment and becomes dim after sometimes.
b) Write the ionic equation of the reaction that took place at the cathode.
Date posted:
April 27, 2017
.
Answers (1)
-
Ammonia gas is used to manufacture nitric (V) acid as shown below.
(Solved)
Ammonia gas is used to manufacture nitric (V) acid as shown below.

i) This process requires the use of a catalyst. In which unit is the catalyst used?
ii) Identify compound A and B.
Date posted:
April 27, 2017
.
Answers (1)
-
A student set up the apparatus as shown in the diagram below to prepare and collect dry
Ammonia gas
(Solved)
A student set up the apparatus as shown in the diagram below to prepare and collect dry
Ammonia gas

i) Identify two mistakes in the set up and give a reason for each mistake.
ii) Name a suitable drying agent for ammonia.
iii) Describe one chemical test for ammonia gas
Date posted:
April 27, 2017
.
Answers (1)
-
Figure 4 shows the heating curve for water.
(Solved)
Figure 4 shows the heating curve for water.
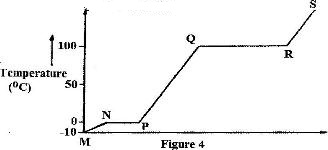
i) Give the names of the intermolecular forces of attraction in the segments:
I. MN
II. RS
ii) The heats of fusion and vaporization of water are 334.4 Jg-1 and 1159.4 g-1 respectively
I. Explain why there is a big difference between the two.
II. How is the difference reflected in the curve?
Date posted:
April 27, 2017
.
Answers (1)
-
The table below shows the pH values of solutions A, B, C and D.
(Solved)
The table below shows the pH values of solutions A, B, C and D.

Select solutions in which a sample of lead (II) hydroxide is likely to dissolve. Give reasons for each solution selected.
Date posted:
April 27, 2017
.
Answers (1)
-
The table below gives the solubilities of substances T and U at 10o C and 40oC
(Solved)
The table below gives the solubilities of substances T and U at 10o C and 40oC

When an aqueous mixture containing 55 g of T and 12 g of U at 80o C was cooled to 10oC, crystals formed.
a) Identify the crystals formed.
b) determine the mass of the crystals formed
c) Name the method used to obtain the crystals
Date posted:
April 27, 2017
.
Answers (1)
-
The curves below show how the electrical conductivity of hydrochloric and ethanoic acids vary with concentration.
(Solved)
The curves below show how the electrical conductivity of hydrochloric and ethanoic acids vary with concentration.
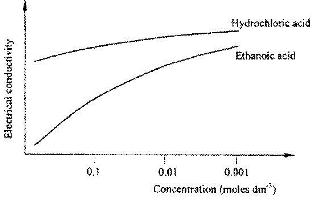
Explain why the electrical conductivity of 0.01 M hydrochloric acid is higher than that of 0.01 M ethanoic acid
Date posted:
April 21, 2017
.
Answers (1)
-
The solubility curve of potassium nitrate is shown below
(Solved)
The solubility curve of potassium nitrate is shown below

a) Determine the solubility of potassium nitrate at 50oC
b) determine the molar concentration of saturated potassium nitrate at 50o(K = 39.0; 0 = 16.0; N = 14.0 and density of water 1g/cm3)
Date posted:
April 21, 2017
.
Answers (1)
-
The graph shows how the pH value of soil in a farm changed over a period of time
(Solved)
The graph shows how the pH value of soil in a farm changed over a period of time
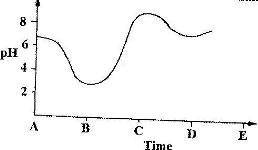
i) Describe how the pH of the soil can be determined
ii) State one factor that may have been responsible for the change in the soil pH in the time interval AB.
Date posted:
April 21, 2017
.
Answers (1)
-
The set-up below was used to demonstrate the effect of heat on hard water
(Solved)
The set-up below was used to demonstrate the effect of heat on hard water
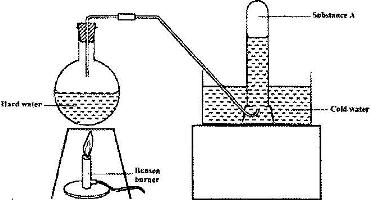
a) Name substance A.
b) Explain why the heating of hard water produced substance A.
Date posted:
April 21, 2017
.
Answers (1)
-
The flow chart below shows the various stages of water treatment. Study it and answer the questions that follow.
(Solved)
The flow chart below shows the various stages of water treatment. Study it and answer the questions that follow.

i) Which substance are likely to be removed in filtration unit I?
ii) What is the name of the process Y?
iii) What is the purpose of:
I. Process Y
II. Addition of sodium hypochlorite
Date posted:
April 21, 2017
.
Answers (1)
-
The table below shows the tests carried out on separate samples of water drawn from a well and the results obtained.
(Solved)
The table below shows the tests carried out on separate samples of water drawn from a well and the results obtained.

a) Identify the cation and the anion present in the water.
b) Write an ionic equation for the reaction which takes place in test (iii).
Date posted:
April 21, 2017
.
Answers (1)