i) J
ii) $Cu_{(s)} \rightarrow Cu^{2+} _{(aq)} + 2e^-$
iii) Sediment
iv) –Used to make coins
-Making alloys such as brass
- Making ornaments
johnmulu answered the question on May 15, 2017 at 09:10
- The diagram below represents a dry cell. Use it to answer the questions that follow.(Solved)
The diagram below represents a dry cell. Use it to answer the questions that follow.

i) Which of the letters represent:
I) Carbon electrode?
II) The electrolyte?
ii) One of the substances used in a dry cell is manganese (IV) oxide. State two roles of manganese (IV) oxide in the dry cell.
Date posted: May 15, 2017. Answers (1)
- The set –up below can be used to produce sodium hydroxide by electrolyzing brine.(Solved)
The set –up below can be used to produce sodium hydroxide by electrolyzing brine.

i) Identify gas Y.
ii) Describe how aqueous sodium hydroxide is formed in the above set-up
iii) One of the uses of sodium hydroxide is in the manufacturing of soaps. State one other use of sodium hydroxide
Date posted: May 15, 2017. Answers (1)
- The set up below was used to investigate the products formed at the electrodes during electrolysis of aqueous magnesium sulphate using inert electrodes. Use it to answer the questions that follow.(Solved)
The set up below was used to investigate the products formed at the electrodes during electrolysis of aqueous magnesium sulphate using inert electrodes. Use it to answer the questions that follow.

i) During the electrolysis hydrogen gas was formed at electrode Y. Identify the anode. Give a reason for your answer.
ii) Why is the concentration of magnesium sulphate expected to increase during electrolysis?
iii) What will be observed if red and blue litmus papers were dipped into the solution after electrolysis?
Date posted: May 15, 2017. Answers (1)
- The apparatus shown in the diagram below were used to investigate the products formed when concentrated sodium chloride was electrolyzed using inert electrodes(Solved)
The apparatus shown in the diagram below were used to investigate the products formed when concentrated sodium chloride was electrolyzed using inert electrodes

a) Write the equation for the reaction that takes place at electrode A.
b) If the concentrated sodium chloride was replaced with dilute sodium chloride, what product would be formed at electrode A? Explain.
Date posted: May 15, 2017. Answers (1)
- The set-below was used by a student to investigate the products formed when aqueous copper (II) chloride was electrolyzed using carbon electrodes(Solved)
The set-below was used by a student to investigate the products formed when aqueous copper (II) chloride was electrolyzed using carbon electrodes

i) Write the equation for the reaction that takes place at the cathode.
ii) Name and describe a chemical test for the product initially formed at the anode when a highly concentrated solution of copper (II) chloride is electrolyzed.
iii) How would the mass of the anode change if the carbon anode was replaced with copper metal? Explain.
Date posted: May 15, 2017. Answers (1)
- The set-up below (Figure 2) was used to electrolyse a bromide of metal D, DBr2.(Solved)
The set-up below (Figure 2) was used to electrolyse a bromide of metal D, DBr2.

a) Write the equation for the reactions at the I. Cathode II. Anode
b) The electrodes used in the experiment were made of carbon and metal D. Which of the two electrodes was used as the anode? Give a reason
c) Give a reason why this experiment is carried out in a fume cupboard.
Date posted: May 15, 2017. Answers (1)
- The diagram below represents a set up that can be used to electrolyze aqueous copper (II) sulphate.(Solved)
The diagram below represents a set up that can be used to electrolyze aqueous copper (II) sulphate.

i) describe s how oxygen gas is produced during the electrolysis.
ii) Explain why copper electrodes are not suitable for this electrolysis.
Date posted: May 15, 2017. Answers (1)
- The diagram below represents an experiment that was set up to investigate movement of ions during electrolysis.(Solved)
The diagram below represents an experiment that was set up to investigate movement of ions during electrolysis.

When the circuit was completed, it was noticed that a blue colour spread towards the right.
a) Explain this observation.
b) Write the equation for the reaction that occurred at the anode.
Date posted: May 15, 2017. Answers (1)
- The diagram below shows the apparatus that can be used to electrolyze acidified water obtain hydrogen and oxygen gases. Study it and answer the questions that follow.(Solved)
The diagram below shows the apparatus that can be used to electrolyze acidified water obtain hydrogen and oxygen gases. Study it and answer the questions that follow.

i) Identify the electrode at which oxidation takes place.
ii) Give a reason why it is necessary to acidify the water.
iii) Explain why hydrochloric acid is not used to acidify the water.
Date posted: May 15, 2017. Answers (1)
- The diagram below represents a diagram cell used to electrolyse pure brine.(Solved)
The diagram below represents a diagram cell used to electrolyse pure brine.

i) Name: I. Product at U
II. Another material that can be used instead of titanium
III. The impurity present in the product at U.
ii) State two functions of the diaphragm
iii) Give one industrial use of the product at U.
Date posted: April 27, 2017. Answers (1)
- The diagram below represents the set-up that was used tom study the effect of an electric current on pure water and dilute sulphuric (VI) acid.(Solved)
The diagram below represents the set-up that was used tom study the effect of an electric current on pure water and dilute sulphuric (VI) acid.

State and explain the observation made when each experiment was started.
Date posted: April 27, 2017. Answers (1)
- The set-up below was used to electrolyse molten lead (II) iodine(Solved)
The set-up below was used to electrolyse molten lead (II) iodine

i) State the observation that was made at the anode during the electrolysis. Give a reason for your answer.
ii) A current of 0.5 A was passed for two hours. Calculate the mass of lead that was deposited (pb = 2071F = 9, 500C)
Date posted: April 27, 2017. Answers (1)
- An electric current was passed through a concentrated solution of copper (II) chloride as shown in the diagram below.(Solved)
An electric current was passed through a concentrated solution of copper (II) chloride as shown in the diagram below.

i) Explain the observation that would be made on the electrolyte as the experiment progresses
ii) Which of the electrodes is the anode? Explain.
Date posted: April 27, 2017. Answers (1)
- The table below gives standard electrode potentials for the metals represented by the letters D, E, F and G. Study it and answer the questions that follow.(Solved)
The table below gives standard electrode potentials for the metals represented by the letters D, E, F and G. Study it and answer the questions that follow.
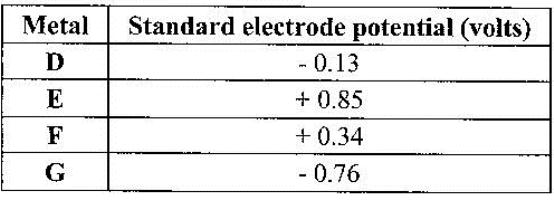
Which metal can be displaced from a solution of its salt by all the other metals in the table? Give a reason.
Date posted: April 27, 2017. Answers (1)
- Study the flow chart below and answer the questions that follow:(Solved)
Study the flow chart below and answer the questions that follow:
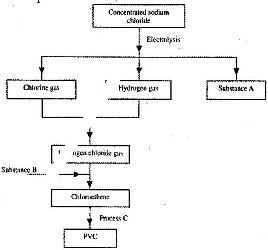
a) Identify substance:
i) A
ii) B
b) Name process C.
c) Give one use of PVC.
Date posted: April 27, 2017. Answers (1)
- The set-up below was used to electroplate a metallic spoon. Study it and answer the questions that follow.(Solved)
The set-up below was used to electroplate a metallic spoon. Study it and answer the questions that follow.
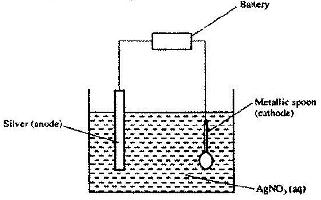
a) Write an ionic equation for the reaction that occurred at the cathode
b) State and explain what happened to the anode.
Date posted: April 27, 2017. Answers (1)
- 100 cm3 of 2 M sulphuric (VI) acid was electrolyzed using the set-up represented by the diagram below(Solved)
100 cm3 of 2 M sulphuric (VI) acid was electrolyzed using the set-up represented by the diagram below
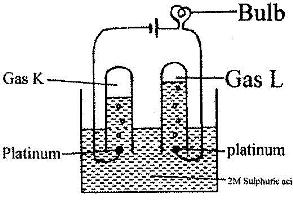
i) Write an equation for the reaction that produces gas L.
ii) Describe how gas K can be identified
iii) Explain the difference in:
I. The volume of the gases produced at the electrodes.
II. Brightness of the bulb if 100 cm3 of 2 M ethanoic acid was used in place of sulphuric (VI) acid.
Date posted: April 27, 2017. Answers (1)
- The diagram below represents a mercury cell that can be used in the industrial manufacture of sodium hydroxide. Study it and answer the questions that follow.(Solved)
The diagram below represents a mercury cell that can be used in the industrial manufacture of sodium hydroxide. Study it and answer the questions that follow.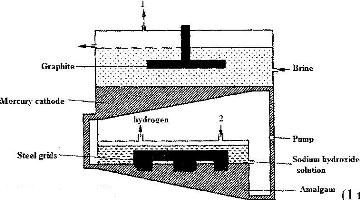
i) Name
I. The raw material introduced at 2
II. another substance that can be used in the cell instead of graphite.
ii) Identify the by – product that comes out at 1
iii) Give
I. One use of sodium hydroxide
II. Two reasons why mercury is recycled.
Date posted: April 27, 2017. Answers (1)
- The set-up below was used during the electrolysis of aqueous magnesium sulphate using inert electrodes.(Solved)
The set-up below was used during the electrolysis of aqueous magnesium sulphate using inert electrodes.

a) Name a suitable pair of electrodes for this experiment.
b) Identify the anions and cations in the solution.
c) Explain the change that occurred to the concentration of magnesium sulphate solution during the experiment
Date posted: April 27, 2017. Answers (1)
- The set –up below was used to electrolyse aqueous copper (II) sulphate.(Solved)
The set –up below was used to electrolyse aqueous copper (II) sulphate.
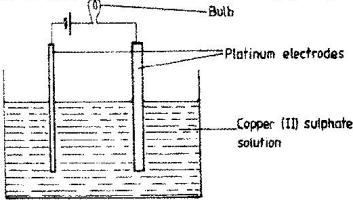
a) Explain why the bulb light brightly at the beginning of the experiment and becomes dim after sometimes.
b) Write the ionic equation of the reaction that took place at the cathode.
Date posted: April 27, 2017. Answers (1)