(i) By changing pressure very slowly or by allowing gas to go to original temperature after the change.
(ii) k represents work done on the gas.
(iii) - Use dry gas
- Make very small changes in temperature.
johnmulu answered the question on May 29, 2017 at 08:31
- Figure 11 shows an insulated cylinder fitted with a pressure gauge, a heating coil and a frictionless piston of cross-sectional area 100 cm2.
(Solved)
Figure 11 shows an insulated cylinder fitted with a pressure gauge, a heating coil and a frictionless piston of cross-sectional area 100 cm2.
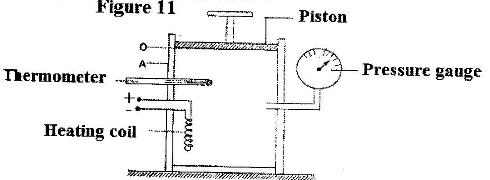
While the piston is at position O, the pressure of the enclosed gas is 10 Ncm-2 at a temperature of 27oC. when a 10 kg mass is placed on the piston, it comes to rest at position A without the temperature of the gas.
(i) Determine the new reading on the pressure gauge.
(ii) State with a reason how the value obtained in (i) compares with the initial pressure.
Date posted: May 29, 2017. Answers (1)
- The tube is now held in a vertical position with the open end facing upwards as shown in figure 8. (Solved)
The tube is now held in a vertical position with the open end facing upwards as shown in figure 8.

Determine:
(i) The pressure of the enclosed air.
(ii) The length (l) of the enclosed air column.
Date posted: May 29, 2017. Answers (1)
- Figure 7 shows a horizontal tube containing air trapped by a mercury thread of length 24 cm. The length of the enclosed air column is 15 cm, The atmospheric pressure is 76 cmHg. (Solved)
Figure 7 shows a horizontal tube containing air trapped by a mercury thread of length 24 cm. The length of the enclosed air column is 15 cm, The atmospheric pressure is 76 cmHg.

State the pressure of the enclosed air.
Date posted: May 29, 2017. Answers (1)
- Figure 6 shows a graph of volume against temperature for a given mass of gas. (Solved)
Figure 6 shows a graph of volume against temperature for a given mass of gas.
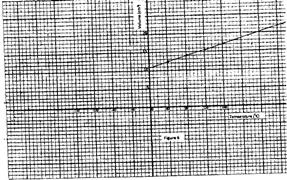
Use the graph to determine the absolute zero temperature in oC
Date posted: May 29, 2017. Answers (1)
- An air bubble is released at the bottom of a tall jar containing a liquid(Solved)
An air bubble is released at the bottom of a tall jar containing a liquid. The height of the liquid column is 80 cm. The volume of the bubble increases from 0.5 cm3 at the bottom of the liquid to 1.15 cm3 at the top. Figure 11 shows the variation of pressure, P, on the bubble with the reciprocal of volume, 15, as it rises in the liquid.
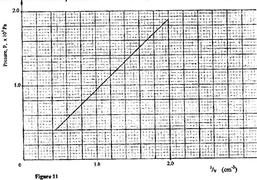
(i) State the reason why the volume increases as the bubble rises in the liquid column.
(ii) From the graph, determine the pressure on the bubble:
(I) at the bottom of the liquid column;
(II) at the top of liquid column
Date posted: May 29, 2017. Answers (1)
- The graph in Figure 11 shows the relationship between volume and temperature for the experiment. (Solved)
The graph in Figure 11 shows the relationship between volume and temperature for the experiment.
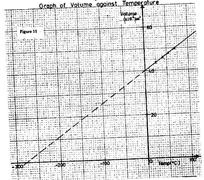
(i) What was the volume of the gas at 0o?
(ii) At what temperature would the volume of the gas be zero?
(iii) Explain why the temperature in part (ii) above cannot be achieved.
Date posted: May 29, 2017. Answers (1)
- Figure 10 shows a set up to investigate the relationship between temperature and volume for a certain gas. (Solved)
Figure 10 shows a set up to investigate the relationship between temperature and volume for a certain gas.
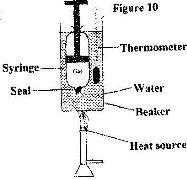
State two factors that are kept constant, in order to determine the relationship.
Date posted: May 29, 2017. Answers (1)
- The graph in figure 7 shows the relationship between the pressure and temperature for an ideal gas. use the information in the figure to answer questions (a)and(b). (Solved)
The graph in figure 7 shows the relationship between the pressure and temperature for an ideal gas. use the information in the figure to answer questions (a)and(b).
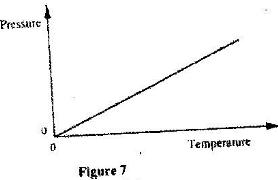
(a) State the unit of the horizontal axis.
(b) Write a statement of the gas law represented by the relationship.
Date posted: May 29, 2017. Answers (1)
- The pressure acting on a gas in a container was changed steadily while the temperature(Solved)
The pressure acting on a gas in a container was changed steadily while the temperature of the gas was maintained constant. The value of volume V of the gas was measured for various values of pressure. The graph in Figure 9 shows the relation between the pressure, P, and reciprocal of volume, \fracf1V
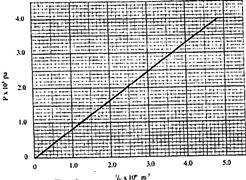
(i) Suggest how the temperature of the gas could be kept constant.
(ii) What physical quantity does k represent?
(iii) State one precaution you would take when performing such an experiment.
Date posted: May 29, 2017. Answers (1)
- Figure 6 shows a simple set up for pressure law apparatus. (Solved)
Figure 6 shows a simple set up for pressure law apparatus.
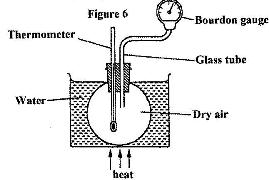
Describe how the apparatus may be used to verify pressure law.
Date posted: May 29, 2017. Answers (1)
- Figure 7 shows an experiment set-up that may be used to investigate one of the laws. The glass tube has a uniform bore and it is graduated in millimeters.(Solved)
Figure 7 shows an experiment set-up that may be used to investigate one of the laws. The glass tube has a uniform bore and it is graduated in millimeters.
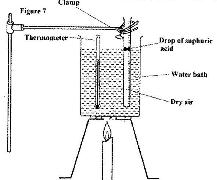
(i) Describe how the experiment is carried out and explain how the results obtained verify the law.
(ii) State two limitations of the set-up.
Date posted: May 29, 2017. Answers (1)
- Figure 5 shows a set up that ,a set up that may be used to verify Charles' law.(Solved)
Figure 5 shows a set up that ,a set up that may be used to verify Charles' law.
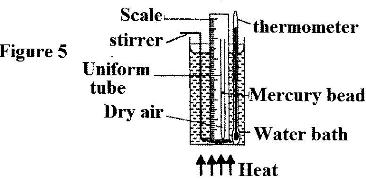
(i) State the measurements that should be taken in the experiment.
(ii) What is the purpose of the water bath?
Date posted: May 29, 2017. Answers (1)
- Fig. 2 shows a set-up that may be used to verify Boyle's law.(Solved)
Fig. 2 shows a set-up that may be used to verify Boyle's law.

Describe the measurements that should be taken in the experiment.
Date posted: May 27, 2017. Answers (1)
- Figure 8 shows a graph of the variation of temperature with time for a pure substance heated at a constant rate. (Solved)
Figure 8 shows a graph of the variation of temperature with time for a pure substance heated at a constant rate.

Assuming that heat transfer to the surroundings is negligible, state the changes observed on the substance in region:
(a) BC;
(b) DE
Date posted: May 27, 2017. Answers (1)
- Figure 8 shows a set-up of apparatus set in an experiment to determine the specific latent of fusion of ice. (Solved)
Figure 8 shows a set-up of apparatus set in an experiment to determine the specific latent of fusion of ice.

The following reading was noted after the heater was switched on for 5 minutes:
- mass of beaker = 130g
- mass of beaker + melted ice = 190 g
(i) Determine the:
(I) Energy supplied by the 60 W heater in the 5 minutes.
(II) Specific latent heat of fusion of ice.
(ii) It was observed that some of the crushed ice melted even before the heater was switched on. State a reason for this observation.
Date posted: May 27, 2017. Answers (1)
- Figure 4 shows a graph of temperature against time when pure melting ice at 0oC is heated uniformly. (Solved)
Figure 4 shows a graph of temperature against time when pure melting ice at 0oC is heated uniformly.

Explain what happens between parts:
(i) OA
(ii) AB
Date posted: May 27, 2017. Answers (1)
- You are provided with the apparatus shown in fig. 9 and a stop watch. (Solved)
You are provided with the apparatus shown in fig. 9 and a stop watch.

Describe an experiment to determine the specific latent heat of steam, l, using the set up. In your answer clearly explain the measurements to be made and how these measurements could be used to determine l.
Date posted: May 27, 2017. Answers (1)
- Figure 11 shows the features of a domestic refrigerator. A volatile liquid circulates through the capillary tubes under the action of the compression pump. (Solved)
Figure 11 shows the features of a domestic refrigerator. A volatile liquid circulates through the capillary tubes under the action of the compression pump.

(i) State the reaction for using a volatile liquid.
(ii) what is the purpose of double wall?
Date posted: May 27, 2017. Answers (1)
- Fig. 11 shows the variation of temperature,θ, with time t,when an immersion heater is used to heat certain liquid. Study the figure and answer questions 22 and 23. (Solved)
Fig. 11 shows the variation of temperature,θ, with time t,when an immersion heater is used to heat certain liquid. Study the figure and answer question.

State the reason for the shape of graph in the section labeled BC
Date posted: May 27, 2017. Answers (1)
- Figure 5 shows the variation of temperature, T (oC), with time, t (seconds) when frozen sea water is heated for some time.
(Solved)
Figure 5 shows the variation of temperature, T (oC), with time, t (seconds) when frozen sea water is heated for some time.

(i) Explain the shape of the curve at the parts labeled A, B and C.
(ii) It is observed that when the temperature starts to rise, the volume initially decreases and then increases. State the reason for this observation.
Date posted: May 27, 2017. Answers (1)