(a) = 234
(b) = 82
johnmulu answered the question on June 6, 2017 at 12:11
- Radium undergoes radioctive decay by emitting an alpha particle to form a daughter nuclide Q as in the reaction:(Solved)
Radium undergoes radioctive decay by emitting an alpha particle to form a daughter nuclide Q as in the reaction:
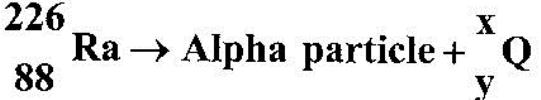
Determine the values of:
(a) x (b) y
Date posted: June 6, 2017. Answers (1)
- The equation below represents a nuclear reaction in which two deuterium nuclei fuse to form Helium and X. (Solved)
The equation below represents a nuclear reaction in which two deuterium nuclei fuse to form Helium and X.
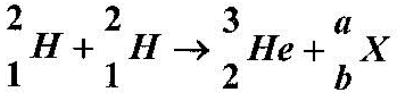
(a) Determine the values of a and b.
(b) Identify X
Date posted: June 6, 2017. Answers (1)
- Figure 6, shows a narrow beam of radiation from a radioctive source, incident to a postcard. The emergent radiation passes through a magnetic field which perpendicular to the plane of the paper, and into the paper. (Solved)
Figure 6, shows a narrow beam of radiation from a radioctive source, incident to a postcard. The emergent radiation passes through a magnetic field which perpendicular to the plane of the paper, and into the paper.

A detector moved along line AC detects radiations only at point B and C. State the two types of radiations detected.
Date posted: June 6, 2017. Answers (1)
- The following is a nuclear equation for a fission process resulting from the reaction of a neutron with a Uranium nucleus. (Solved)
The following is a nuclear equation for a fission process resulting from the reaction of a neutron with a Uranium nucleus.
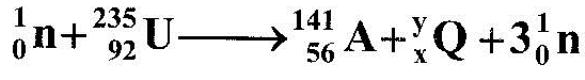
(i) Determine the values of of x and y
(ii) State the source of the energy released.
(iii) Explain how this reaction is made continuous in a nuclear reactor
Date posted: June 6, 2017. Answers (1)
- Figure 11 shows the path of radiation from a radioactive source. The field is perpendicular to the paper and directed out of the paper. (Solved)
Figure 11 shows the path of radiation from a radioactive source. The field is perpendicular to the paper and directed out of the paper.
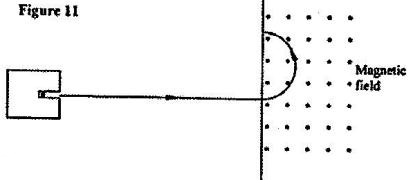
(a)Identify the radiation.
(b) (i) State the effect of the radiation on the gas inside the Geiger-Muller tube.
(ii) Explain how the large discharge current is created.
(c) The following is a nuclear equation for a fission process resulting from the reaction of a neutron with a Uranium nucleus.
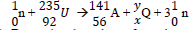
(i) Determine the values of x and y.
ii) State the source of the energy released.
(iii)Explain how this reaction is made continuous in a nuclear reactor.
Date posted: June 6, 2017. Answers (1)
- A radioactive isotope of copper decays to form an isotope of Zinc as shown below. (Solved)
A radioactive isotope of copper decays to form an isotope of Zinc as shown below.
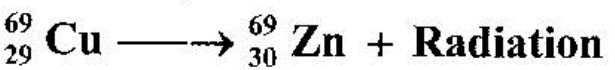
Name the radiation emitted and give a reason for your answer.
Date posted: June 6, 2017. Answers (1)
- The following part of radioactive decay series
(Solved)
The following part of radioactive decay series
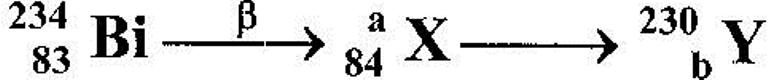
Determine the values of a and b
Date posted: June 6, 2017. Answers (1)
- A nuclear reaction is represented by the following equation. (Solved)
A nuclear reaction is represented by the following equation.
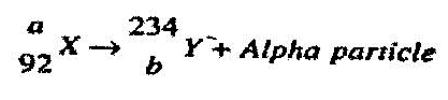
Determine the values of a and b
Date posted: June 6, 2017. Answers (1)
- The following represents a nuclear reaction involving the nuclide polonium Po. (Solved)
The following represents a nuclear reaction involving the nuclide polonium Po.
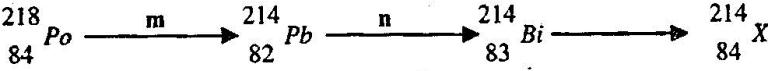
Identify m, n and X
Date posted: June 6, 2017. Answers (1)
- Figure 4 shows the cross-section of a diffusion cloud chamber used to detect radiation from radioctive sources. (Solved)
Figure 4 shows the cross-section of a diffusion cloud chamber used to detect radiation from radioctive sources.
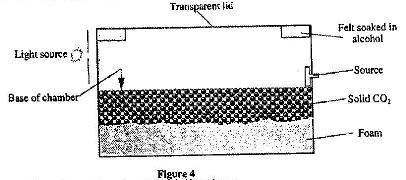
(i) State one function of each of the following:
Alcohol
Solid CO2
(ii) When radiation from the source enters the chamber, some white traces are observed. Explain how these traces are formed and state how the radiation is identified.
(iii) A leaf electroscope can also be used as a detector of radiation. State two advantages of the diffusion cloud chamber over the leaf electroscope as a detector.
Date posted: June 6, 2017. Answers (1)
- Figure 4 shows a Geiger Muller (G.M,) tube. (Solved)
Figure 4 shows a Geiger Muller (G.M,) tube.
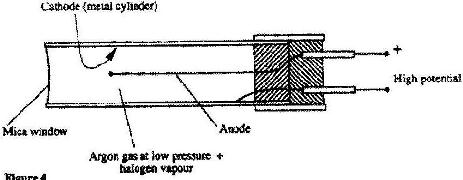
(i) Give the reason why the mica window is made thin
(ii) Explain how the radiation entering the tube through the window is detected by the tube.
(iii) What is the purpose of the halogen vapour?
Date posted: June 6, 2017. Answers (1)
- The following equation shows part of a radioactive decay process. (Solved)
The following equation shows part of a radioactive decay process.
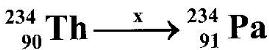
Name the radiation x
Date posted: June 6, 2017. Answers (1)
- Figure 3 shows a device for producing metal foils of constant thickness.(Solved)
Figure 3 shows a device for producing metal foils of constant thickness. Any change in thickness can be detected by the Geiger tube and recorded by the Geiger counter. The pressure exerted by the roller is then adjusted to keep the thickness constant.
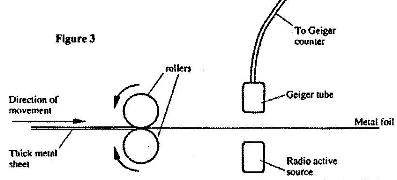
(i) State the change in the metal foil that will lead to a decrease in the Geiger counter reading.
(ii) Give a reason for your answer in (i) above.
(iii) State the change in the roller pressure that should be made as a result of this decrease in the Geiger counter reading.
(vi) Give a reason for answer in (iii) above.
Date posted: June 6, 2017. Answers (1)
- Below is a nuclear reaction. (Solved)
Below is a nuclear reaction.
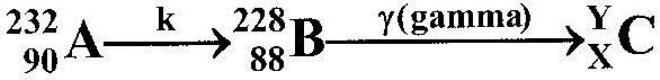
(i) Identify radiation k.
(ii) Determine the value of X and Y.
Date posted: June 6, 2017. Answers (1)
- Figure 2 shows the path of radiation from a radioctive source after entering a magnetic field. The magnetic field is directed into the paper and is perpendicular to the plane of the paper as shown in the figure. (Solved)
Figure 2 shows the path of radiation from a radioctive source after entering a magnetic field. The magnetic field is directed into the paper and is perpendicular to the plane of the paper as shown in the figure.
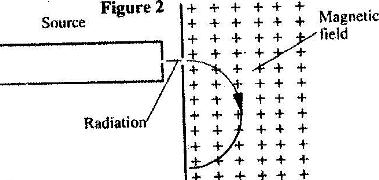
Identify the radiation. Give a reason for your answer.
Date posted: June 6, 2017. Answers (1)
- The graph in Fig.20 shows the disintegration per second versus time in seconds, s, for as sample of radioactive material(Solved)
The graph in Fig.20 shows the disintegration per second versus time in seconds, s, for as sample of radioactive material
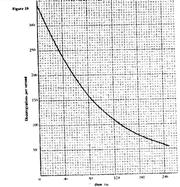
Determine the half-life of the sample.
Date posted: June 6, 2017. Answers (1)
- Figure 6 shows a graph of the variation of the number of atoms of a certain radioctive material with time. (Solved)
Figure 6 shows a graph of the variation of the number of atoms of a certain radioctive material with time.
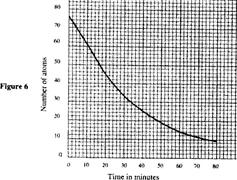
Determine the half-life of the material.
Date posted: June 6, 2017. Answers (1)
- Figure 8 shows ultra-violet light striking a polished zinc plate placed on a negatively charged gold-leaf electroscope. (Solved)
Figure 8 shows ultra-violet light striking a polished zinc plate placed on a negatively charged gold-leaf electroscope.

Explain the following observations.
(i) The leaf of the electroscope falls.
(ii) When the same experiment was repeated with a positively charged electroscope the leaf did not fall
Date posted: June 6, 2017. Answers (1)
- In figure 18 ultra-violet (u.v.) light falls on a zinc plate placed on a charged leaf electroscope. It is observed that the leaf collapses. (Solved)
In figure 18 ultra-violet (u.v.) light falls on a zinc plate placed on a charged leaf electroscope. It is observed that the leaf collapses.

Explain how this observation may be used to determine the type of charge on the electroscope.
Date posted: June 6, 2017. Answers (1)
- Explain how land and sea breeze occurs(Solved)
Explain how land and sea breeze occurs.
Date posted: June 6, 2017. Answers (1)