Add acidified potassium manganate (VII) solution to both separately. CH3C=CCH2CH3 will turn potassium manganate (VII) from purple to colourless while CH3C(CH3)2CH3 will have no effect.
johnmulu answered the question on June 7, 2017 at 07:52
- Give the names of the following compounds:(Solved)
Give the names of the following compounds:
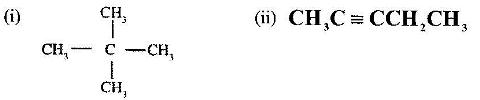
Date posted: June 7, 2017. Answers (1)
- Use the flow chart below to answer the questions that follow. (Solved)
Use the flow chart below to answer the questions that follow.
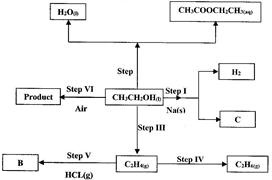
(i) Name:
(I) The type of reaction that occurs in step II;
(II) Substance B.
(ii) Give the formula of substance C.
Date posted: June 7, 2017. Answers (1)
- The table below gives the formula of four compounds L, M, N and P. (Solved)
The table below gives the formula of four compounds L, M, N and P.
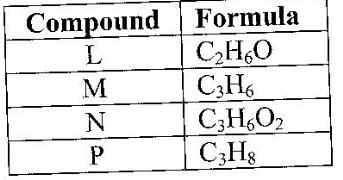
Giving a reason in each case, select the letter which represents a compound that:
(i) Decolorizes bromine in the absence of UV light.
(ii) Gives effervescence when reacted with aqueous sodium carbonate.
Date posted: June 7, 2017. Answers (1)
- Study the flow chart below and answer the questions that follow. (Solved)
Study the flow chart below and answer the questions that follow.
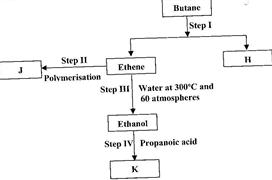
(i) State the conditions for the reaction in Step I to occur.
(ii) Identify substance J.
(iii) State one disadvantage of the continued use of substance such as J.
Date posted: June 7, 2017. Answers (1)
- The structure below represents a sweet smelling compound.(Solved)
The structure below represents a sweet smelling compound.
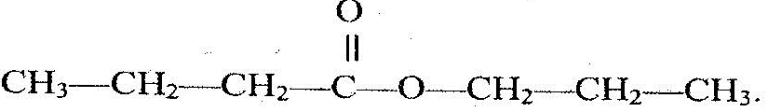
Give the name of two organic compounds that can be used to prepare this compound in the laboratory.
Date posted: June 7, 2017. Answers (1)
- The structure of a detergent is. (Solved)
The structure of a detergent is.
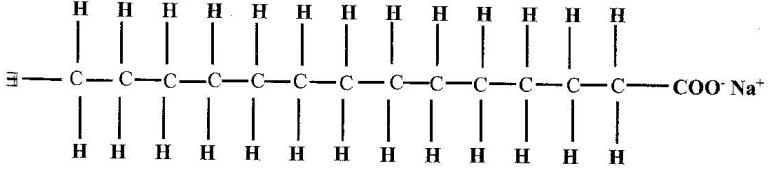
(a) Write the molecular formular of the detergent.
(b) What type of detergent is represented by the formula?
Date posted: June 7, 2017. Answers (1)
- Ethanol obtained from glucose can be converted to ethene as shown below;(Solved)
Ethanol obtained from glucose can be converted to ethene as shown below;
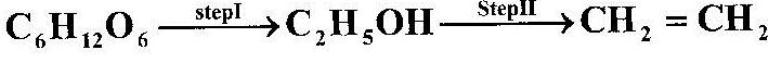
Name and describe the process that takes place in steps I and II.
Date posted: June 7, 2017. Answers (1)
- The table below shows the relative molecular masses and the boiling points of pentane and propan-1-o1(Solved)
The table below shows the relative molecular masses and the boiling points of pentane and propan-1-o1

Explain why the boiling point of propan-1-ol is higher than that of pentane.
Date posted: May 25, 2017. Answers (1)
- Propane can be changed into methane and ethane as shown in the equation below. (Solved)
Propane can be changed into methane and ethane as shown in the equation below.

Name the process undergone by propane
Date posted: May 25, 2017. Answers (1)
- Study the flow chart below and answer the questions that follow
(Solved)
Study the flow chart below and answer the questions that follow

(i) Identify reagent L.
(ii) Name the catalyst used in step 5.
(iii) What name is given to the process that takes place in step 5?
Date posted: May 25, 2017. Answers (1)
- Study the information in the table below and answer the questions that follow. (Solved)
Study the information in the table below and answer the questions that follow.

Give a reason why the difference in the molar heats of combustion between successive alcohols are close.
Date posted: May 25, 2017. Answers (1)
- The flow chart below shows a series of reactions starting with ethanol. Study it and answer the questions that follow. (Solved)
The flow chart below shows a series of reactions starting with ethanol. Study it and answer the questions that follow.

(i) Name: I. Process A. II. Substances B and C
(ii) Explain why it is necessary to use high pressure to change gas B into the polymer.
Date posted: May 25, 2017. Answers (1)
- The structures below represents a portion of a polymer. (Solved)
The structures below represents a portion of a polymer.

Give:
(a) The name of the polymer.
(b) One industrial use of the polymer.
Date posted: May 25, 2017. Answers (1)
- Study the scheme given below and answer the questions that follow. (Solved)
Study the scheme given below and answer the questions that follow.

(i) Name processes I and II
(ii) Identify the products A and B.
(iii) Name one catalyst used in process II.
Date posted: May 25, 2017. Answers (1)
- Ethane and Ethene react with bromine according to the equation given below. (Solved)
Ethane and Ethene react with bromine according to the equation given below.
(i) C2H6(g)+Br2(g)→C2H5Br2(g)+HBr(g)
(ii) C2H4(g)+Br2(g)→C2H4Br2(l)
Name the type of bromination reaction that takes place in: (i) and (ii).
Date posted: May 25, 2017. Answers (1)
- Give the names of the following compounds (Solved)
Give the names of the following compounds

Date posted: May 25, 2017. Answers (1)
- The scheme below was used to prepare a cleansing agent. Study it and answer the questions that follow. (Solved)
The scheme below was used to prepare a cleansing agent. Study it and answer the questions that follow.

(i) What name is given to the type of cleansing agent prepared by the method shown in the scheme?
(ii) Name one chemical substance added in step II.
(iii) What is the purpose of adding the chemical substance named in (ii) above.
(iv) Name one other suitable substance that can be used in step I
(v) Give one advantage of using the cleansing agent above.
Date posted: May 25, 2017. Answers (1)
- Study the scheme given above and answer the questions that follow. (Solved)
Study the scheme given above and answer the questions that follow.

(i) Name the reagents used in:
Step I.... Step II.. Step IV ...
(ii) Explain one disadvantage of the continued use of items made from the compound formed in step III.
Date posted: May 25, 2017. Answers (1)
- The structure shown below represent two cleansing agents, A and B.(Solved)
The structure shown below represent two cleansing agents, A and B.

Which cleansing agent would be more suitable for washing in water containing magnesium sulphate? Give a reason.
Date posted: May 25, 2017. Answers (1)
- Study the table below and answer the questions that follow:(Solved)
Study the table below and answer the questions that follow:

(i) Which of the compounds is a solid at 10.0 o C? Explain.
(ii) Choose two compounds which are members of the same homologous series and explain the difference in their melting points.
(iii) The compound C3H8O is an alcohol. How does its solubility in water differ from the solubility of C5H12 in water? Explain.
Date posted: May 25, 2017. Answers (1)