7. Heat lost by metal = 0.2 × C × (100 - 21)
Heat gained by H2O = 0.42 × 4200 (25 - 21)
0.2 × C × (100 - 21)
0.42 × 4200 × (25 - 21)
0.2 × C × 79 = 0.42 × 4200 × 4
C = 446.58 J/ kg
sharon kalunda answered the question on March 25, 2019 at 09:14
- When a particular substance at a certain temperature is heated, it expands. When the same substance at the same temperature is cooled, it also expands....(Solved)
When a particular substance at a certain temperature is heated, it expands. When the same substance at the same temperature is cooled, it also expands.
a) What is the substance?
b) What is the temperature?
Date posted: March 25, 2019. Answers (1)
- In the evening the inside of green houses may be seen to have water droplets on them. Why does this happen?(Solved)
In the evening the inside of green houses may be seen to have water droplets on them. Why does this happen?
Date posted: March 25, 2019. Answers (1)
- The diagram below shows apparatus used to observe the behaviour of smoke particle in air.(Solved)
The diagram below shows apparatus used to observe the behaviour of smoke particle in air.
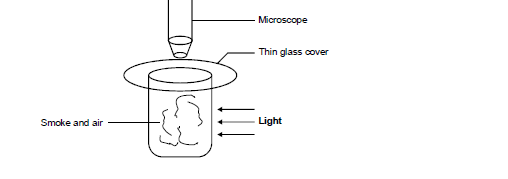
i) Why are smoke particles suitable for use in this experiment.
ii) What does the experiment tell you about the behaviour of the air molecules in the cell?
iii) What difference if any would be seen in the motion of the smoke particles if a weaker light was used.
Date posted: March 25, 2019. Answers (1)
- The figure below shows a soft iron core placed between poles of two magnets. Copy the diagram and sketch the magnetic field pattern(Solved)
The figure below shows a soft iron core placed between poles of two magnets. Copy the diagram and sketch the magnetic field pattern
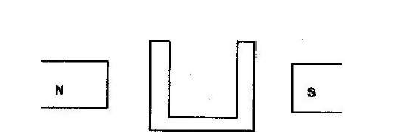
Date posted: March 25, 2019. Answers (1)
- The figure below shows a soft iron ring placed between the poles of magnet. Copy the diagram and sketch the magnetic field pattern(Solved)
The figure below shows a soft iron ring placed between the poles of magnet. Copy the diagram and sketch the magnetic field pattern
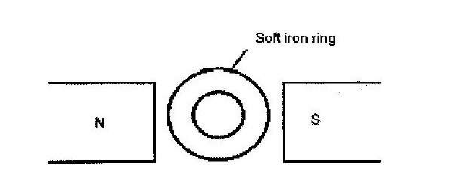
Date posted: March 25, 2019. Answers (1)
- In figure below, the arrow indicates the direction of the current in the conductor. Sketch on the diagram the magnetic field pattern(Solved)
In figure below, the arrow indicates the direction of the current in the conductor. Sketch on the diagram the magnetic field pattern
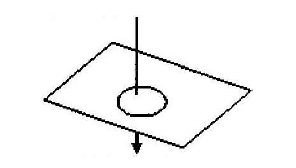
Date posted: March 25, 2019. Answers (1)
- Figure below shows a wire XY at right angles to a magnetic field. XY is part of a circuit containing a galvanometer. When XY is...(Solved)
Figure below shows a wire XY at right angles to a magnetic field. XY is part of a circuit containing a galvanometer. When XY is moved, the current flows in the direction shown. State the direction in which XY is moved.
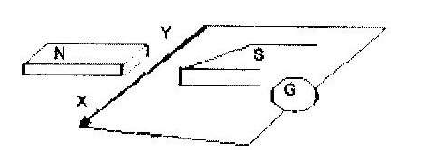
Date posted: March 25, 2019. Answers (1)
- The figure below shows two parallel current- carrying conductors A and B placed close to each other. The direction of the current is into the...(Solved)
The figure below shows two parallel current- carrying conductors A and B placed close to each other. The direction of the current is into the plane of the paper. Copy the diagram and on the same figure;
(i) Sketch the magnetic field pattern
(ii) indicate the force F due to the current on each conductor
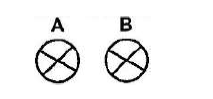
Date posted: March 25, 2019. Answers (1)
- The figure below represents a long horizontal insulated wire AB connected to an electric circuit. A plotting compass is placed on the wire as shown. When...(Solved)
The figure below represents a long horizontal insulated wire AB connected to an electric circuit. A plotting compass is placed on the wire as shown. When the switch K is closed, the plotting compass shows a deflection. State two changes which can be made in the circuit to increase the deflection.
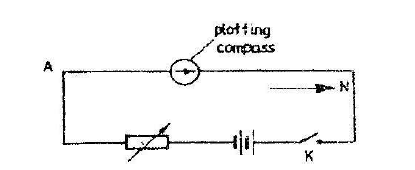
Date posted: March 25, 2019. Answers (1)
- Give two differences between uniform and non-uniform magnetic fields(Solved)
Give two differences between uniform and non-uniform magnetic fields
Date posted: March 25, 2019. Answers (1)
- One way of demagnetizing bar is to place it in a solenoid in which an alternating current (ac) flows. How is the demagnetization achieved?(Solved)
One way of demagnetizing bar is to place it in a solenoid in which an alternating current (ac) flows. How is the demagnetization achieved?
Date posted: March 25, 2019. Answers (1)
- You are provided with two iron bars, X and Y, one is magnetized and the other is not. Explain how you would identify the magnetized...(Solved)
You are provided with two iron bars, X and Y, one is magnetized and the other is not. Explain how you would identify the magnetized bar without using a magnet.
Date posted: March 25, 2019. Answers (1)
- The figure shows a wire in a magnetic field. A current is switched on to flow through the wire in the direction shown. State the...(Solved)
The figure shows a wire in a magnetic field. A current is switched on to flow through the wire in the direction shown. State the direction of motion of the wire.
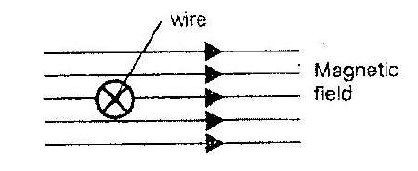
Date posted: March 25, 2019. Answers (1)
- The graph in the figure below shows the relationship between the attractive forces of an electromagnetic and the magnetizing current. Give reasons for the shape...(Solved)
The graph in the figure below shows the relationship between the attractive forces of an electromagnetic and the magnetizing current. Give reasons for the shape of the curve in terms of the domain theory.
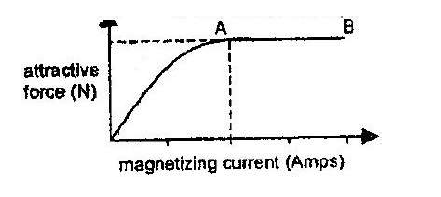
Date posted: March 25, 2019. Answers (1)
- When ammeter is connected between the two plates of a simple cell, the pointer deflects along the scale. Explain(Solved)
When ammeter is connected between the two plates of a simple cell, the pointer deflects along the scale. Explain
Date posted: March 23, 2019. Answers (1)
- Explain clearly the precautionary measures you would take to maintain the efficiency of an accumulator?(Solved)
Explain clearly the precautionary measures you would take to maintain the efficiency of an accumulator?
Date posted: March 23, 2019. Answers (1)
- State the advantage of Nickel-cadmium battery over the lead -acid type(Solved)
State the advantage of Nickel-cadmium battery over the lead -acid type
Date posted: March 23, 2019. Answers (1)
- A student wishes to investigate the relationship between current and voltage for a certain device X. In the space provided, draw a circuit diagram including...(Solved)
A student wishes to investigate the relationship between current and voltage for a certain device X. In the space provided, draw a circuit diagram including two cells, rheostat, ammeter, voltmeter and the device X that would be suitable in obtaining the desired results.
Date posted: March 23, 2019. Answers (1)
- A current of 0.08A passes in a circuit for 2.5 minutes. How much charge passes through a point in the circuit?(Solved)
A current of 0.08A passes in a circuit for 2.5 minutes. How much charge passes through a point in the circuit?
Date posted: March 23, 2019. Answers (1)
- What current will a 500O resistor connected to a source of 240V draw?(Solved)
What current will a 500Ω resistor connected to a source of 240V draw?
Date posted: March 23, 2019. Answers (1)