(i)The solution inside the gas jar rose to take up the space which was occupied by the active part of air.
(ii)The candle went out because in a fixed amount of air only a part of it was used up called the ‘active air’ with the rest ‘Inactive air’ which doesn’t support combustion.
Kavungya answered the question on March 25, 2019 at 09:22
- Describe an experiment to show that Carbon dioxide is available in the atmosphere.(Solved)
Describe an experiment to show that Carbon dioxide is available in the atmosphere.
Date posted: March 25, 2019. Answers (1)
- What evidence is there to show that there is water vapour in the air?(Solved)
What evidence is there to show that there is water vapour in the air?
Date posted: March 25, 2019. Answers (1)
- What is air?(Solved)
What is air?
Date posted: March 25, 2019. Answers (1)
- What is the difference between the melting point of a pure substance and that of an impure substance?(Solved)
What is the difference between the melting point of a pure substance and that of an impure substance?
Date posted: March 25, 2019. Answers (1)
- Below is an arrangement which was used to prepare gas.
(i) Which one property of gas X can you tell from the method of preparation?
(ii) Identify...(Solved)
Below is an arrangement which was used to prepare gas.
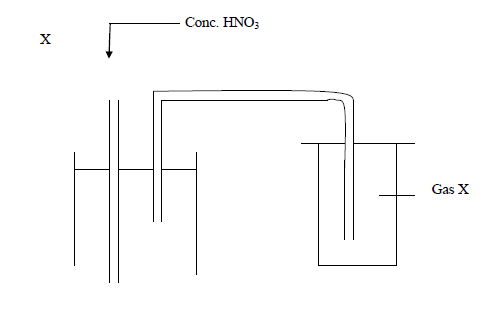
(i) Which one property of gas X can you tell from the method of preparation?
(ii) Identify gas X
(iii) Why is Lead (II) nitrate more suitable than other nitrates for the preparation of gas X?
Date posted: March 25, 2019. Answers (1)
- What principle do refrigerators work on?(Solved)
What principle do refrigerators work on?
Date posted: March 25, 2019. Answers (1)
- Briefly explain how ice-cream vendors use solid carbon dioxide (dry ice) to keep the ice-cream frozen.(Solved)
Briefly explain how ice-cream vendors use solid carbon dioxide (dry ice) to keep the ice-cream frozen.
Date posted: March 25, 2019. Answers (1)
- Using Kinetic theory of matter explain condensation process.(Solved)
Using Kinetic theory of matter explain condensation process.
Date posted: March 25, 2019. Answers (1)
- Why is that gases don’t have definite shape?(Solved)
Why is that gases don’t have definite shape?
Date posted: March 25, 2019. Answers (1)
- How do you identify pure substances in chemistry?(Solved)
How do you identify pure substances in chemistry?
Date posted: March 25, 2019. Answers (1)
- The following are some of the methods used to collect gases. With reasons state which method is suitable for each of the following gases, Ammonia,...(Solved)
The following are some of the methods used to collect gases. With reasons state which method is suitable for each of the following gases, Ammonia, carbon dioxide, oxygen.
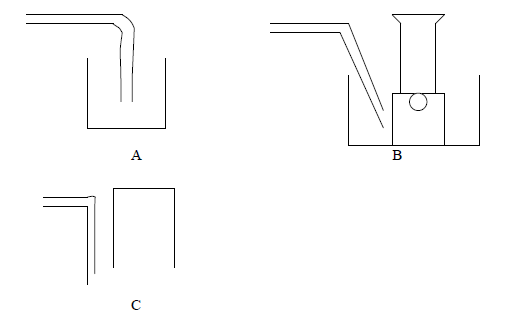
Date posted: March 25, 2019. Answers (1)
- Explain sublimation using Kinetic theory of matter.(Solved)
Explain sublimation using Kinetic theory of matter.
Date posted: March 25, 2019. Answers (1)
- What does Kinetic theory of matter state?(Solved)
What does Kinetic theory of matter state?
Date posted: March 25, 2019. Answers (1)
- What is the difference between a compound and a mixture?(Solved)
What is the difference between a compound and a mixture?
Date posted: March 25, 2019. Answers (1)
- Distinguish between physical and chemical changes.(Solved)
Distinguish between physical and chemical changes.
Date posted: March 25, 2019. Answers (1)
- Describe briefly how filtration is applied industrially.(Solved)
Describe briefly how filtration is applied industrially.
Date posted: March 25, 2019. Answers (1)
- The time – temperature graph obtained when ice was heated to its boiling point is given below.
(i) Describe the changes along regions A, B, C...(Solved)
The time – temperature graph obtained when ice was heated to its boiling point is given below.
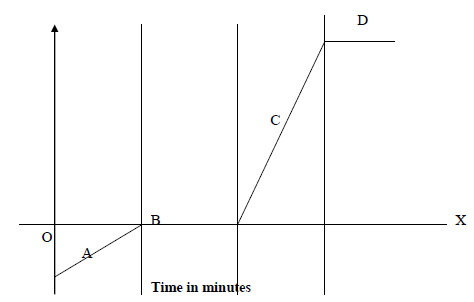
(i) Describe the changes along regions A, B, C and D.
(ii) Explain why both B and D levelled off?
Date posted: March 25, 2019. Answers (1)
- Where in Kenya is Crystallization method used? State what happens briefly.(Solved)
Where in Kenya is Crystallization method used? State what happens briefly.
Date posted: March 25, 2019. Answers (1)
- When fine chalk is suspended in water and viewed through a microscope the chalk particles appear to move in a random fashion. This motion is...(Solved)
When fine chalk is suspended in water and viewed through a microscope the chalk particles appear to move in a random fashion. This motion is a result of?
Date posted: March 25, 2019. Answers (1)
- State how chromatography is applied in real life situation.(Solved)
State how chromatography is applied in real life situation.
Date posted: March 25, 2019. Answers (1)