When magnesium is burned in air, it chemically combines with oxygen gas to form a compound called magnesium oxide which is heavier than the metal i.e. the white ash is heavier than the original piece of magnesium.
Thus when an element is heated or burnt in air, it combines with oxygen from air and gains in weight.
Kavungya answered the question on March 25, 2019 at 09:23
- Study the experiment below and answer the questions that follow.
The above experiment was carried to study what happens when candle burns in a fixed amount...(Solved)
Study the experiment below and answer the questions that follow.
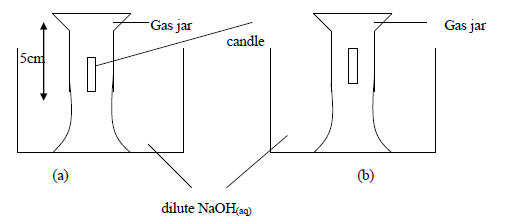
The above experiment was carried to study what happens when candle burns in a fixed amount of air. After burning of the candle the NaOH(aq) level in the jar rose(b) and the candle went off.
(i) Why do you think the solution level rose inside the gas jar, filling only a part of it?
(ii) Why did the candle go out after burning only for a while?
Date posted: March 25, 2019. Answers (1)
- Describe an experiment to show that Carbon dioxide is available in the atmosphere.(Solved)
Describe an experiment to show that Carbon dioxide is available in the atmosphere.
Date posted: March 25, 2019. Answers (1)
- What evidence is there to show that there is water vapour in the air?(Solved)
What evidence is there to show that there is water vapour in the air?
Date posted: March 25, 2019. Answers (1)
- What is air?(Solved)
What is air?
Date posted: March 25, 2019. Answers (1)
- What is the difference between the melting point of a pure substance and that of an impure substance?(Solved)
What is the difference between the melting point of a pure substance and that of an impure substance?
Date posted: March 25, 2019. Answers (1)
- Below is an arrangement which was used to prepare gas.
(i) Which one property of gas X can you tell from the method of preparation?
(ii) Identify...(Solved)
Below is an arrangement which was used to prepare gas.
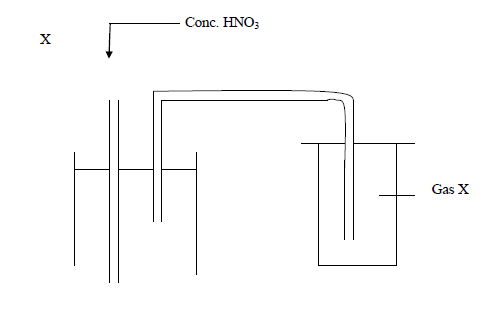
(i) Which one property of gas X can you tell from the method of preparation?
(ii) Identify gas X
(iii) Why is Lead (II) nitrate more suitable than other nitrates for the preparation of gas X?
Date posted: March 25, 2019. Answers (1)
- What principle do refrigerators work on?(Solved)
What principle do refrigerators work on?
Date posted: March 25, 2019. Answers (1)
- Briefly explain how ice-cream vendors use solid carbon dioxide (dry ice) to keep the ice-cream frozen.(Solved)
Briefly explain how ice-cream vendors use solid carbon dioxide (dry ice) to keep the ice-cream frozen.
Date posted: March 25, 2019. Answers (1)
- Using Kinetic theory of matter explain condensation process.(Solved)
Using Kinetic theory of matter explain condensation process.
Date posted: March 25, 2019. Answers (1)
- Why is that gases don’t have definite shape?(Solved)
Why is that gases don’t have definite shape?
Date posted: March 25, 2019. Answers (1)
- How do you identify pure substances in chemistry?(Solved)
How do you identify pure substances in chemistry?
Date posted: March 25, 2019. Answers (1)
- The following are some of the methods used to collect gases. With reasons state which method is suitable for each of the following gases, Ammonia,...(Solved)
The following are some of the methods used to collect gases. With reasons state which method is suitable for each of the following gases, Ammonia, carbon dioxide, oxygen.
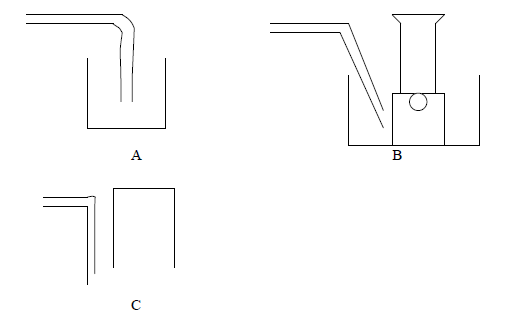
Date posted: March 25, 2019. Answers (1)
- Explain sublimation using Kinetic theory of matter.(Solved)
Explain sublimation using Kinetic theory of matter.
Date posted: March 25, 2019. Answers (1)
- What does Kinetic theory of matter state?(Solved)
What does Kinetic theory of matter state?
Date posted: March 25, 2019. Answers (1)
- What is the difference between a compound and a mixture?(Solved)
What is the difference between a compound and a mixture?
Date posted: March 25, 2019. Answers (1)
- Distinguish between physical and chemical changes.(Solved)
Distinguish between physical and chemical changes.
Date posted: March 25, 2019. Answers (1)
- Describe briefly how filtration is applied industrially.(Solved)
Describe briefly how filtration is applied industrially.
Date posted: March 25, 2019. Answers (1)
- The time – temperature graph obtained when ice was heated to its boiling point is given below.
(i) Describe the changes along regions A, B, C...(Solved)
The time – temperature graph obtained when ice was heated to its boiling point is given below.
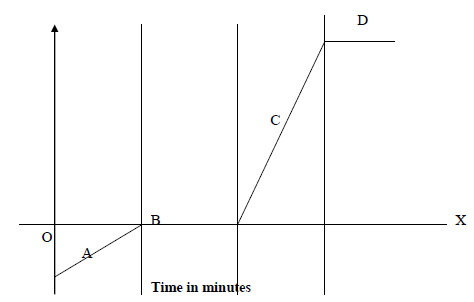
(i) Describe the changes along regions A, B, C and D.
(ii) Explain why both B and D levelled off?
Date posted: March 25, 2019. Answers (1)
- Where in Kenya is Crystallization method used? State what happens briefly.(Solved)
Where in Kenya is Crystallization method used? State what happens briefly.
Date posted: March 25, 2019. Answers (1)
- When fine chalk is suspended in water and viewed through a microscope the chalk particles appear to move in a random fashion. This motion is...(Solved)
When fine chalk is suspended in water and viewed through a microscope the chalk particles appear to move in a random fashion. This motion is a result of?
Date posted: March 25, 2019. Answers (1)