- Distinguish between acidic and basic oxides.(Solved)
Distinguish between acidic and basic oxides.
Date posted: March 25, 2019. Answers (1)
- What appropriate measures are being taken to reduce atmospheric pollution?(Solved)
What appropriate measures are being taken to reduce atmospheric pollution?
Date posted: March 25, 2019. Answers (1)
- What gases are a common pollutant in the atmosphere?(Solved)
What gases are a common pollutant in the atmosphere?
Date posted: March 25, 2019. Answers (1)
- Describe an experiment to investigate the “active part of air”.(Solved)
Describe an experiment to investigate the “active part of air”.
Date posted: March 25, 2019. Answers (1)
- 1.(a) List some methods used to prevent rusting.
(b) Name one substance which speeds up the rusting process(Solved)
1.(a) List some methods used to prevent rusting.
(b) Name one substance which speeds up the rusting process
Date posted: March 25, 2019. Answers (1)
- What are the conditions necessary for rusting?(Solved)
What are the conditions necessary for rusting?
Date posted: March 25, 2019. Answers (1)
- Why is there any increase when a metal like magnesium is burned in air?(Solved)
Why is there any increase when a metal like magnesium is burned in air?
Date posted: March 25, 2019. Answers (1)
- Study the experiment below and answer the questions that follow.
The above experiment was carried to study what happens when candle burns in a fixed amount...(Solved)
Study the experiment below and answer the questions that follow.
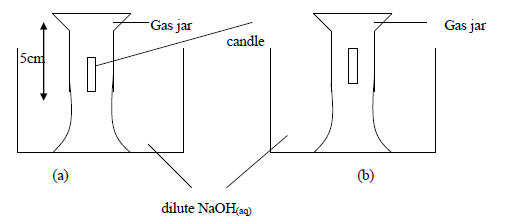
The above experiment was carried to study what happens when candle burns in a fixed amount of air. After burning of the candle the NaOH(aq) level in the jar rose(b) and the candle went off.
(i) Why do you think the solution level rose inside the gas jar, filling only a part of it?
(ii) Why did the candle go out after burning only for a while?
Date posted: March 25, 2019. Answers (1)
- Describe an experiment to show that Carbon dioxide is available in the atmosphere.(Solved)
Describe an experiment to show that Carbon dioxide is available in the atmosphere.
Date posted: March 25, 2019. Answers (1)
- What evidence is there to show that there is water vapour in the air?(Solved)
What evidence is there to show that there is water vapour in the air?
Date posted: March 25, 2019. Answers (1)
- What is air?(Solved)
What is air?
Date posted: March 25, 2019. Answers (1)
- What is the difference between the melting point of a pure substance and that of an impure substance?(Solved)
What is the difference between the melting point of a pure substance and that of an impure substance?
Date posted: March 25, 2019. Answers (1)
- Below is an arrangement which was used to prepare gas.
(i) Which one property of gas X can you tell from the method of preparation?
(ii) Identify...(Solved)
Below is an arrangement which was used to prepare gas.
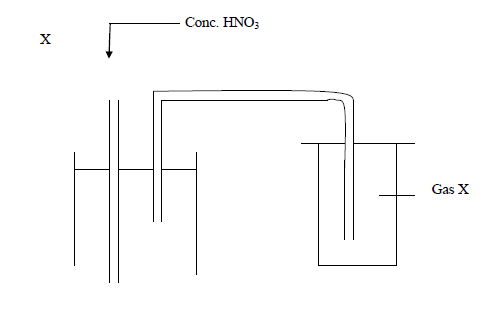
(i) Which one property of gas X can you tell from the method of preparation?
(ii) Identify gas X
(iii) Why is Lead (II) nitrate more suitable than other nitrates for the preparation of gas X?
Date posted: March 25, 2019. Answers (1)
- What principle do refrigerators work on?(Solved)
What principle do refrigerators work on?
Date posted: March 25, 2019. Answers (1)
- Briefly explain how ice-cream vendors use solid carbon dioxide (dry ice) to keep the ice-cream frozen.(Solved)
Briefly explain how ice-cream vendors use solid carbon dioxide (dry ice) to keep the ice-cream frozen.
Date posted: March 25, 2019. Answers (1)
- Using Kinetic theory of matter explain condensation process.(Solved)
Using Kinetic theory of matter explain condensation process.
Date posted: March 25, 2019. Answers (1)
- Why is that gases don’t have definite shape?(Solved)
Why is that gases don’t have definite shape?
Date posted: March 25, 2019. Answers (1)
- How do you identify pure substances in chemistry?(Solved)
How do you identify pure substances in chemistry?
Date posted: March 25, 2019. Answers (1)
- The following are some of the methods used to collect gases. With reasons state which method is suitable for each of the following gases, Ammonia,...(Solved)
The following are some of the methods used to collect gases. With reasons state which method is suitable for each of the following gases, Ammonia, carbon dioxide, oxygen.
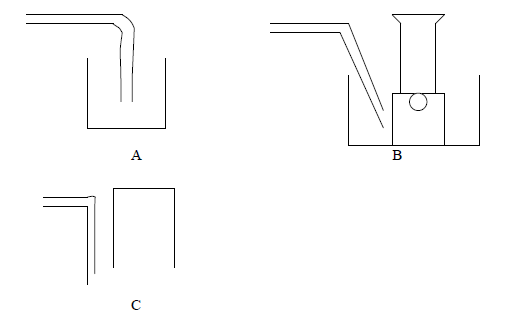
Date posted: March 25, 2019. Answers (1)
- Explain sublimation using Kinetic theory of matter.(Solved)
Explain sublimation using Kinetic theory of matter.
Date posted: March 25, 2019. Answers (1)