Air is a mixture which can be separated by physical means – fractional distillation, water is a compound of oxygen and hydrogen and these two can’t be separated by distillation but by a chemical means like electrolysis.
Kavungya answered the question on March 25, 2019 at 10:36
- How is hydrogen manufactured on a large scale? Give three industrial uses of the gas.(Solved)
How is hydrogen manufactured on a large scale? Give three industrial uses of the gas.
Date posted: March 25, 2019. Answers (1)
- Below are the main sources of water pollution. Explain how each of them affects the environment?
(i) Sewage
(ii) Fertilisers
(iii) Chemicals/Pesticides
(iv) Oil and detergents(Solved)
Below are the main sources of water pollution. Explain how each of them affects the environment?
(i) Sewage
(ii) Fertilisers
(iii) Chemicals/Pesticides
(iv) Oil and detergents
Date posted: March 25, 2019. Answers (1)
- The diagram below represents a paper chromatogram of two sugars A and B.
State one property of A that makes it move faster than B towards...(Solved)
The diagram below represents a paper chromatogram of two sugars A and B.
State one property of A that makes it move faster than B towards the solvent front.
Date posted: March 25, 2019. Answers (1)
- An experiment was set up as shown in the diagram below.
Explain what is observed after a few days.(Solved)
An experiment was set up as shown in the diagram below.
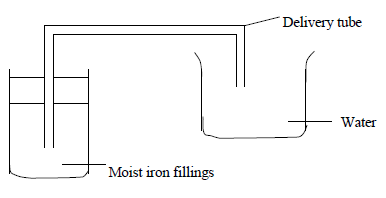
Explain what is observed after a few days.
Date posted: March 25, 2019. Answers (1)
- In an experiment, dry hydrogen chloride gas was passed through heated Zinc turnings as shown in the diagram below. The gas produced was then passed...(Solved)
In an experiment, dry hydrogen chloride gas was passed through heated Zinc turnings as shown in the diagram below. The gas produced was then passed through heated Lead II Oxide.
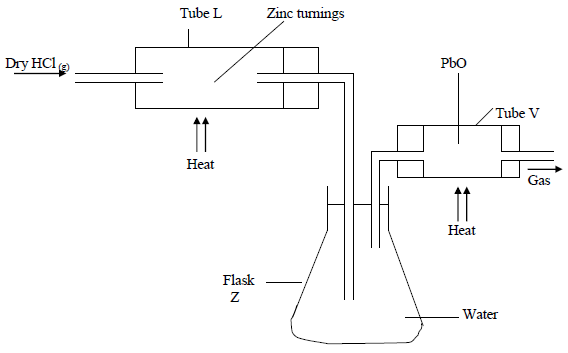
(i) What is the function of the water in the flask?
(ii) Write equations for the reactions that took place in the tubes.
(iii) How would the total mass of tube V and its contents compare before and after the experiment? Explain.
Date posted: March 25, 2019. Answers (1)
- Paper chromatography of a plant extract gave the following results.
Which Solvent is the most suitable for purifying the extract? Explain.(Solved)
Paper chromatography of a plant extract gave the following results.
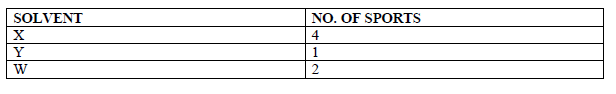
Which Solvent is the most suitable for purifying the extract? Explain.
Date posted: March 25, 2019. Answers (1)
- A form one pupil lit a Bunsen burner with its air hole fully open. The regions of the flames were labelled as shown below.
(a) Name...(Solved)
A form one pupil lit a Bunsen burner with its air hole fully open. The regions of the flames were labelled as shown below.

(a) Name part (A)
(b) Which region is the hottest
(c) Which colour is region C?
Date posted: March 25, 2019. Answers (1)
- Carbon dioxide doesn’t support combustion, yet burning of magnesium ribbon introduced in jar of carbon dioxide continues to burn. Explain this.(Solved)
Carbon dioxide doesn’t support combustion, yet burning of magnesium ribbon introduced in jar of carbon dioxide continues to burn. Explain this.
Date posted: March 25, 2019. Answers (1)
- Where is the competition for oxygen reaction applied industrially?(Solved)
Where is the competition for oxygen reaction applied industrially?
Date posted: March 25, 2019. Answers (1)
- Pollution is a health hazard which is directly proportional to the level of industrialisation in developing countries. Give evidence to justify this proclamation.(Solved)
Pollution is a health hazard which is directly proportional to the level of industrialisation in developing countries. Give evidence to justify this proclamation.
Date posted: March 25, 2019. Answers (1)
- An experiment on competition for oxygen was carried out and results were tabulated in the table below. Use information in the table to answer the...(Solved)
An experiment on competition for oxygen was carried out and results were tabulated in the table below. Use information in the table to answer the questions that follow.

(i) What is the best conclusion that can be drawn on the basis of the above results?
(ii) What is the order of reactivity of elements in this experiment?
(iii) What are the products formed where a reaction occurred?
(iv) If you were given an oxide copper how would you obtain copper metal from it?
Date posted: March 25, 2019. Answers (1)
- Distinguish between acidic and basic oxides.(Solved)
Distinguish between acidic and basic oxides.
Date posted: March 25, 2019. Answers (1)
- What appropriate measures are being taken to reduce atmospheric pollution?(Solved)
What appropriate measures are being taken to reduce atmospheric pollution?
Date posted: March 25, 2019. Answers (1)
- What gases are a common pollutant in the atmosphere?(Solved)
What gases are a common pollutant in the atmosphere?
Date posted: March 25, 2019. Answers (1)
- Describe an experiment to investigate the “active part of air”.(Solved)
Describe an experiment to investigate the “active part of air”.
Date posted: March 25, 2019. Answers (1)
- 1.(a) List some methods used to prevent rusting.
(b) Name one substance which speeds up the rusting process(Solved)
1.(a) List some methods used to prevent rusting.
(b) Name one substance which speeds up the rusting process
Date posted: March 25, 2019. Answers (1)
- What are the conditions necessary for rusting?(Solved)
What are the conditions necessary for rusting?
Date posted: March 25, 2019. Answers (1)
- Why is there any increase when a metal like magnesium is burned in air?(Solved)
Why is there any increase when a metal like magnesium is burned in air?
Date posted: March 25, 2019. Answers (1)
- Study the experiment below and answer the questions that follow.
The above experiment was carried to study what happens when candle burns in a fixed amount...(Solved)
Study the experiment below and answer the questions that follow.
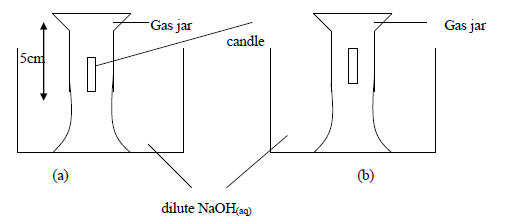
The above experiment was carried to study what happens when candle burns in a fixed amount of air. After burning of the candle the NaOH(aq) level in the jar rose(b) and the candle went off.
(i) Why do you think the solution level rose inside the gas jar, filling only a part of it?
(ii) Why did the candle go out after burning only for a while?
Date posted: March 25, 2019. Answers (1)
- Describe an experiment to show that Carbon dioxide is available in the atmosphere.(Solved)
Describe an experiment to show that Carbon dioxide is available in the atmosphere.
Date posted: March 25, 2019. Answers (1)