a)(i) A – yellow
(ii) B – red
b)Hydroxide ions are removed and the solution becomes acidic.
Kavungya answered the question on March 25, 2019 at 10:59
- List five methods used to prepare salts in the laboratory regardless of whether the salts are soluble or insoluble.(Solved)
List five methods used to prepare salts in the laboratory regardless of whether the salts are soluble or insoluble.
Date posted: March 25, 2019. Answers (1)
- What is neutralization?(Solved)
What is neutralization?
Date posted: March 25, 2019. Answers (1)
- State the uses of four alkaline solutions.(Solved)
State the uses of four alkaline solutions.
Date posted: March 25, 2019. Answers (1)
- State how you can determine the strength of acids and alkalis.(Solved)
State how you can determine the strength of acids and alkalis.
Date posted: March 25, 2019. Answers (1)
- Why is it better to use indicators rather than the sense of taste when testing for acids and alkalis?(Solved)
Why is it better to use indicators rather than the sense of taste when testing for acids and alkalis?
Date posted: March 25, 2019. Answers (1)
- Define the following terms:
i)Acids
ii)Bases
iii)Salts
iv)Indicators(Solved)
Define the following terms:
i)Acids
ii)Bases
iii)Salts
iv)Indicators
Date posted: March 25, 2019. Answers (1)
- Explain briefly the following observations about a sample of hard water (a) when boiled it formed some white precipitate (b) even after boiling the water...(Solved)
Explain briefly the following observations about a sample of hard water (a) when boiled it formed some white precipitate (b) even after boiling the water formed scum with soap (c) Sodium Carbonate made the water completely soft.
Date posted: March 25, 2019. Answers (1)
- Name the materials used to prepare soaps and soapless detergents.(Solved)
Name the materials used to prepare soaps and soapless detergents.
Date posted: March 25, 2019. Answers (1)
- You are given a clear liquid in a beaker. Explain how you would confirm that the liquid is pure water.(Solved)
You are given a clear liquid in a beaker. Explain how you would confirm that the liquid is pure water.
Date posted: March 25, 2019. Answers (1)
- Oxygen and nitrogen are obtained from air by fractional distillation. Why can this method be used for this purpose yet cannot be used to obtained...(Solved)
Oxygen and nitrogen are obtained from air by fractional distillation. Why can this method be used for this purpose yet cannot be used to obtained hydrogen and oxygen from water?
Date posted: March 25, 2019. Answers (1)
- How is hydrogen manufactured on a large scale? Give three industrial uses of the gas.(Solved)
How is hydrogen manufactured on a large scale? Give three industrial uses of the gas.
Date posted: March 25, 2019. Answers (1)
- Below are the main sources of water pollution. Explain how each of them affects the environment?
(i) Sewage
(ii) Fertilisers
(iii) Chemicals/Pesticides
(iv) Oil and detergents(Solved)
Below are the main sources of water pollution. Explain how each of them affects the environment?
(i) Sewage
(ii) Fertilisers
(iii) Chemicals/Pesticides
(iv) Oil and detergents
Date posted: March 25, 2019. Answers (1)
- The diagram below represents a paper chromatogram of two sugars A and B.
State one property of A that makes it move faster than B towards...(Solved)
The diagram below represents a paper chromatogram of two sugars A and B.
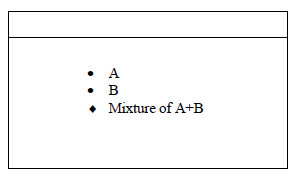
State one property of A that makes it move faster than B towards the solvent front.
Date posted: March 25, 2019. Answers (1)
- An experiment was set up as shown in the diagram below.
Explain what is observed after a few days.(Solved)
An experiment was set up as shown in the diagram below.
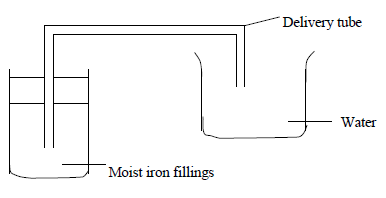
Explain what is observed after a few days.
Date posted: March 25, 2019. Answers (1)
- In an experiment, dry hydrogen chloride gas was passed through heated Zinc turnings as shown in the diagram below. The gas produced was then passed...(Solved)
In an experiment, dry hydrogen chloride gas was passed through heated Zinc turnings as shown in the diagram below. The gas produced was then passed through heated Lead II Oxide.
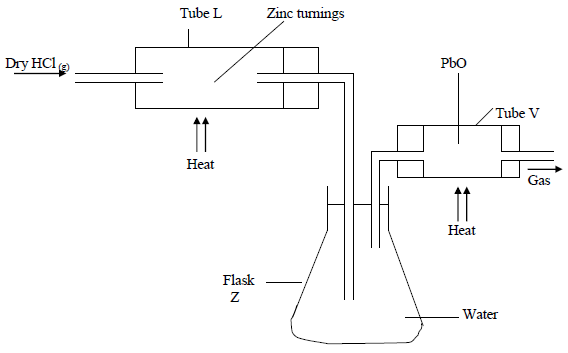
(i) What is the function of the water in the flask?
(ii) Write equations for the reactions that took place in the tubes.
(iii) How would the total mass of tube V and its contents compare before and after the experiment? Explain.
Date posted: March 25, 2019. Answers (1)
- Paper chromatography of a plant extract gave the following results.
Which Solvent is the most suitable for purifying the extract? Explain.(Solved)
Paper chromatography of a plant extract gave the following results.
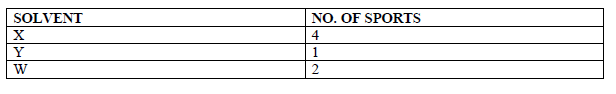
Which Solvent is the most suitable for purifying the extract? Explain.
Date posted: March 25, 2019. Answers (1)
- A form one pupil lit a Bunsen burner with its air hole fully open. The regions of the flames were labelled as shown below.
(a) Name...(Solved)
A form one pupil lit a Bunsen burner with its air hole fully open. The regions of the flames were labelled as shown below.

(a) Name part (A)
(b) Which region is the hottest
(c) Which colour is region C?
Date posted: March 25, 2019. Answers (1)
- Carbon dioxide doesn’t support combustion, yet burning of magnesium ribbon introduced in jar of carbon dioxide continues to burn. Explain this.(Solved)
Carbon dioxide doesn’t support combustion, yet burning of magnesium ribbon introduced in jar of carbon dioxide continues to burn. Explain this.
Date posted: March 25, 2019. Answers (1)
- Where is the competition for oxygen reaction applied industrially?(Solved)
Where is the competition for oxygen reaction applied industrially?
Date posted: March 25, 2019. Answers (1)
- Pollution is a health hazard which is directly proportional to the level of industrialisation in developing countries. Give evidence to justify this proclamation.(Solved)
Pollution is a health hazard which is directly proportional to the level of industrialisation in developing countries. Give evidence to justify this proclamation.
Date posted: March 25, 2019. Answers (1)