- The piston moves outwards / the piston moves towards the left.
- As the temperature increases, kinetic energy of the gas molecules increases and this increases the rate of collision between the gas molecules and walls of the container. This causes pressure of the gas to increase thus pushing the piston outwards.
sharon kalunda answered the question on March 26, 2019 at 08:26
-
Figure below shows air molecules in front of a hollow wooden box P set vibrating by a tuning fork T of frequency 800Hz.
(Solved)
Figure below shows air molecules in front of a hollow wooden box P set vibrating by a tuning fork T of frequency 800Hz.
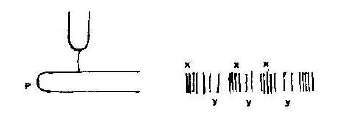
(a) What is the purpose of fixing the tuning fork on the box which is open on one end?
(b) Name the section labeled X and Y
(c) State and explain the nature of the waves shown
(d) Given that the speed of sound in air is 330ms-1. Calculate the wavelength of the waves.
Date posted:
March 26, 2019
.
Answers (1)
-
Differentiate between displacement and distance.
(Solved)
Differentiate between displacement and distance.
Date posted:
March 26, 2019
.
Answers (1)
-
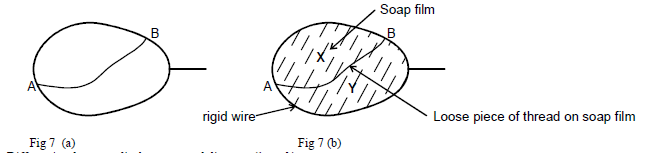
Figure 7(a) below shows a loop of a wire with a string tied loosely at point A and B. When the loop was dipped into...
(Solved)
Figure 7(a) below shows a loop of a wire with a string tied loosely at point A and B. When the loop was dipped into a soap solution and then moved out a soap film is formed as shown. In fig 7(b) below. State and hence explain the observation made when side X of the film is broken.
Date posted:
March 26, 2019
.
Answers (1)
-
Describe an experiment to show that sound cannot travel in a vacuum.
(Solved)
Describe an experiment to show that sound cannot travel in a vacuum.
Date posted:
March 26, 2019
.
Answers (1)
-
Figure below shows an inverted funnel placed above a light ball.
(Solved)
Figure below shows an inverted funnel placed above a light ball.

Fast moving air is then blown through the neck of the funnel. State and explain the observation made.
Date posted:
March 26, 2019
.
Answers (1)
-
The work done in stretching a spring by 50mm is given as 0.08J. Calculate the spring constant.
(Solved)
The work done in stretching a spring by 50mm is given as 0.08J. Calculate the spring constant.
Date posted:
March 26, 2019
.
Answers (1)
-
A gun is fired and an echo heard at the same place 0.5s later. How far is the barrier, which reflected the sound from the...
(Solved)
A gun is fired and an echo heard at the same place 0.5s later. How far is the barrier, which reflected the sound from the gun? (Speed of sound 330m/s).
Date posted:
March 26, 2019
.
Answers (1)
-
Figure below shows a thick copper plate that is very hot, one side is black and the other is shiny. Two thermometers are placed at...
(Solved)
Figure below shows a thick copper plate that is very hot, one side is black and the other is shiny. Two thermometers are placed at the same distance from each side as shown.

Neglecting heat loss to the surrounding, state with a reason which thermometer records a higher temperature after 10 minutes.
Date posted:
March 26, 2019
.
Answers (1)
-
Two identical sources of sound S1 and S2 are emitting the same frequency. Explain with reasons, the observations that will be made by an observe...
(Solved)
Two identical sources of sound S1 and S2 are emitting the same frequency. Explain with reasons, the observations that will be made by an observe listening to the sound emitted who was moving slowly along the lines, PQ and MN

Date posted:
March 26, 2019
.
Answers (1)
-
State the reason why steel is normally used to reinforce concrete in construction other than aluminium
(Solved)
State the reason why steel is normally used to reinforce concrete in construction other than aluminium
Date posted:
March 26, 2019
.
Answers (1)
-
When a sound wave travels from a dense to a less dense gas, its velocity changes. What wave property does this observation show?
(Solved)
When a sound wave travels from a dense to a less dense gas, its velocity changes. What wave property does this observation show?
Date posted:
March 26, 2019
.
Answers (1)
-
Explain why the rate of diffusion of a gas decreases with decrease in temperature.
(Solved)
Explain why the rate of diffusion of a gas decreases with decrease in temperature.
Date posted:
March 26, 2019
.
Answers (1)
-
State the type of wave produced when a stretched string is plucked
(Solved)
State the type of wave produced when a stretched string is plucked.
Date posted:
March 26, 2019
.
Answers (1)
-
Figure below shows a U tube manometer containing two liquids. Given that h1 and h2 are 22cm and 19cm respectively,find the density of the liquid...
(Solved)
Figure below shows a U tube manometer containing two liquids. Given that h1 and h2 are 22cm and 19cm respectively,find the density of the liquid Z. Give your answer to 2 significant figures.

Date posted:
March 26, 2019
.
Answers (1)
-
Best FM station broadcasts on a frequency of 250 KHz and the wavelength of its signals is 1200m.
(Solved)
Best FM station broadcasts on a frequency of 250 KHz and the wavelength of its signals is 1200m.
Calculate:
(i) The speed of radio waves in m/s
(ii) The wavelength of the signal of another station that broadcasts on a frequency of 200KHZ.
Date posted:
March 26, 2019
.
Answers (1)
-
The figure below shows a beaker containing molten candle wax. Indicate on the same diagram the position of the centre of gravity. When the candle...
(Solved)
The figure below shows a beaker containing molten candle wax. Indicate on the same diagram the position of the centre of gravity. When the candle wax solidifies.

Date posted:
March 26, 2019
.
Answers (1)
-
Give an example to demonstrate that waves carry energy
(Solved)
Give an example to demonstrate that waves carry energy
Date posted:
March 26, 2019
.
Answers (1)
-
Give an example which show that speed of a wave depends on the medium in which it travels.
(Solved)
Give an example which show that speed of a wave depends on the medium in which it travels.
Date posted:
March 26, 2019
.
Answers (1)
-
State ONE factor that does not change as water moves from shallow to deep part.
(Solved)
State ONE factor that does not change as water moves from shallow to deep part.
Date posted:
March 26, 2019
.
Answers (1)
-
Figure below represents a system in equilibrium.
(Solved)
Figure below represents a system in equilibrium.

Determine the force F needed to maintain the equilibrium.
Date posted:
March 26, 2019
.
Answers (1)