i) To ensure that the temperature of the furnace is maintained by the solid material / to ensure minimum heat loss to the surroundings during transfer.
ii) - Error while reading the thermometer
- Error while taking reading of the mass.
- There is heat lost to the surrounding during transfer.
sharon kalunda answered the question on March 26, 2019 at 08:55
- Define specific heat capacity.(Solved)
Define specific heat capacity.
Date posted: March 26, 2019. Answers (1)
- Sketch a velocity- time graph showing the motion of a ball vertically upwards with an initial velocity of u.(Solved)
Sketch a velocity- time graph showing the motion of a ball vertically upwards with an initial velocity of u.
Date posted: March 26, 2019. Answers (1)
- The diagram below shows part of the motion of a tennis ball, which is projected vertically upwards from the ground and allowed to bounce on...(Solved)
The diagram below shows part of the motion of a tennis ball, which is projected vertically upwards from the ground and allowed to bounce on the ground. Use this information to answer questions that follow.
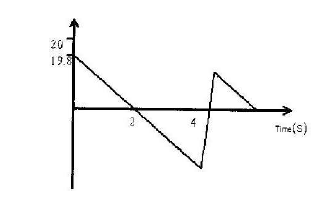
a) Describe the motion of the ball relating it to different positions of the ball along the following AB, BC, CDE.
b) From the graph, calculate the acceleration due to gravity.
c) How high does the ball rise initially?
d) Explain why E is not at the same level as A.
Date posted: March 26, 2019. Answers (1)
- The figure below shows a loading ramp of length 8m and height 2m. Bags weighing 1000N each are conveyed from point O to P along...(Solved)
The figure below shows a loading ramp of length 8m and height 2m. Bags weighing 1000N each are conveyed from point O to P along the plane. Each bag experiences a friction force of 50N.
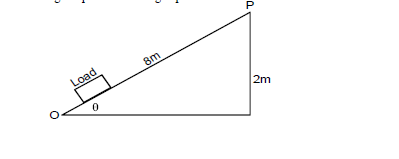
Show that the velocity ratio is given by 1/Sin 0
Date posted: March 26, 2019. Answers (1)
- Figure below shows a pulley system used to lift a load.(Solved)
Figure below shows a pulley system used to lift a load.
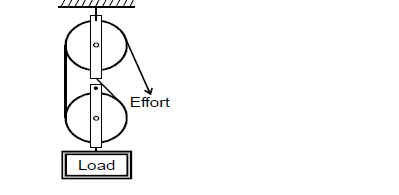
Determine the velocity ratio.
Date posted: March 26, 2019. Answers (1)
- Water flows along a horizontal pipe of cross sectional area 30cm2. The speed of the water is 4m/s but it reaches 7.5m/s in a constriction...(Solved)
Water flows along a horizontal pipe of cross sectional area 30cm2. The speed of the water is 4m/s but it reaches 7.5m/s in a constriction in the pipe. Calculate the area of the constriction.
Date posted: March 26, 2019. Answers (1)
- An object moving at 26m/s starts to accelerate at 2m/s² so that its velocity becomes 48m/s. Find
i) The distance moved during this acceleration.
ii) The...(Solved)
An object moving at 26m/s starts to accelerate at 2m/s² so that its velocity becomes 48m/s. Find
i) The distance moved during this acceleration.
ii) The object is now braked so that it comes to rest in a time of 12 seconds. Find the braking force if its mass was 27000g.
Date posted: March 26, 2019. Answers (1)
- Figure below shows velocity time graphs for two objects A and B drawn on the same axes.(Solved)
Figure below shows velocity time graphs for two objects A and B drawn on the same axes.
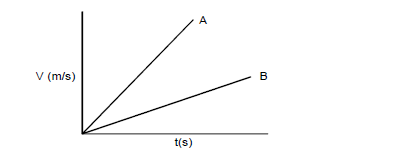
State with a reason which of the two objects stops in a shorter distance when the same size of force in applied against each given that they are of equal masses.
Date posted: March 26, 2019. Answers (1)
- A pupil blows a current of air over the surface of a sheet of paper held close to its mouth. State and explain what happens...(Solved)
A pupil blows a current of air over the surface of a sheet of paper held close to its mouth. State and explain what happens to the paper.
Date posted: March 26, 2019. Answers (1)
- The figure below shows water forces through a hydraulic system by a pump. An air chamber is used to maintain a continuous flow of water...(Solved)
The figure below shows water forces through a hydraulic system by a pump. An air chamber is used to maintain a continuous flow of water during both the up-stroke and down-stroke of the piston pump.
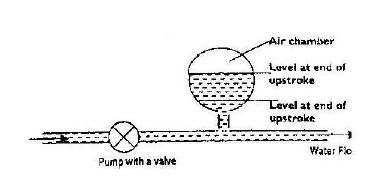
Explain how the continuous flow of water is maintained.
Date posted: March 26, 2019. Answers (1)
- Figure below shows block A placed on a bench connected with a piece of thread through a pulley to a pan B where masses are...(Solved)
Figure below shows block A placed on a bench connected with a piece of thread through a pulley to a pan B where masses are being added.
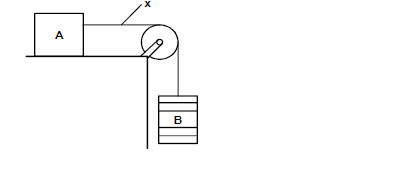
State a condition that must be met for block A to slide towards the right.
Date posted: March 26, 2019. Answers (1)
- When spraying a field of water using a hose pipe, it is common to reduce the pipes opening in order to spray water furthest. Other...(Solved)
When spraying a field of water using a hose pipe, it is common to reduce the pipes opening in order to spray water furthest. Other than pressure, what other quality is varied in the process?
Date posted: March 26, 2019. Answers (1)
- Distinguish between the following terms
(i) Intensity and loudness
(ii) Frequency and pitch(Solved)
Distinguish between the following terms
(i) Intensity and loudness
(ii) Frequency and pitch
Date posted: March 26, 2019. Answers (1)
- Sound is very faint in high altitudes than at sea level. Why?(Solved)
Sound is very faint in high altitudes than at sea level. Why?
Date posted: March 26, 2019. Answers (1)
- Figure below shows a gas enclosed in a container.(Solved)
Figure below shows a gas enclosed in a container.
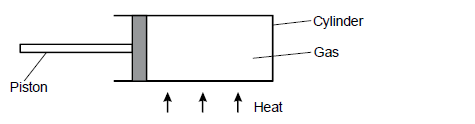
State and explain using the kinetic theory of matter. What will happen to the piston when the cylinder is heated.
Date posted: March 26, 2019. Answers (1)
- Figure below shows air molecules in front of a hollow wooden box P set vibrating by a tuning fork T of frequency 800Hz.(Solved)
Figure below shows air molecules in front of a hollow wooden box P set vibrating by a tuning fork T of frequency 800Hz.
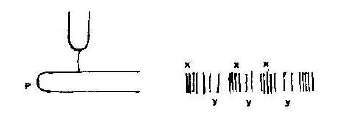
(a) What is the purpose of fixing the tuning fork on the box which is open on one end?
(b) Name the section labeled X and Y
(c) State and explain the nature of the waves shown
(d) Given that the speed of sound in air is 330ms-1. Calculate the wavelength of the waves.
Date posted: March 26, 2019. Answers (1)
- Differentiate between displacement and distance.(Solved)
Differentiate between displacement and distance.
Date posted: March 26, 2019. Answers (1)
- Figure 7(a) below shows a loop of a wire with a string tied loosely at point A and B. When the loop was dipped into...(Solved)
Figure 7(a) below shows a loop of a wire with a string tied loosely at point A and B. When the loop was dipped into a soap solution and then moved out a soap film is formed as shown. In fig 7(b) below. State and hence explain the observation made when side X of the film is broken.
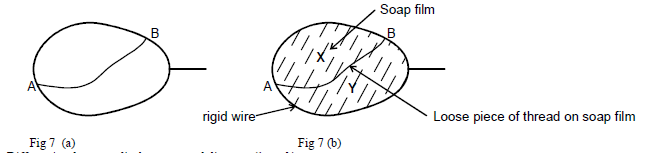
Date posted: March 26, 2019. Answers (1)
- Describe an experiment to show that sound cannot travel in a vacuum.(Solved)
Describe an experiment to show that sound cannot travel in a vacuum.
Date posted: March 26, 2019. Answers (1)
- Figure below shows an inverted funnel placed above a light ball.(Solved)
Figure below shows an inverted funnel placed above a light ball.

Fast moving air is then blown through the neck of the funnel. State and explain the observation made.
Date posted: March 26, 2019. Answers (1)