a)
= -0.44 + +1.66
= +1.22v
b) G, E, F
c) Yes- G cannot be displaced the E2+ ions because it is less reactive than E.?
sharon kalunda answered the question on March 26, 2019 at 13:09
-
The table below gives some properties of three elements X,Y and Z.
(Solved)
The table below gives some properties of three elements X,Y and Z.
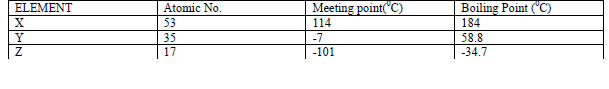
(a) Which element is in liquid form at room temperature? Give reason.
(b) Explain why the boiling point of element X is higher than that of element Z.
Date posted:
March 26, 2019
.
Answers (1)
-
The diagram below is a cross section of a dry cell. Study it and answer the questions that follow.
(Solved)
The diagram below is a cross section of a dry cell. Study it and answer the questions that follow.

(i) Write the equation for the reaction in which electrons are produced.
(ii) The Zinc can is lined with Ammonium Chloride and Zinc Chloride paste. What would happen if the mixture was to become dry? Give reason.
Date posted:
March 26, 2019
.
Answers (1)
-
Study the flow chart below and answer the questions that follow.
(Solved)
Study the flow chart below and answer the questions that follow.

i) Name the process in step I and the conditions for the reaction in step I
Name of process
Conditions
ii) Identify substance N
iii) Give
I. One disadvantage of the continued use of substance such as M
II. The name of the process that takes place in step III
III. The name and structural formula of the substance Q
Name
Structural formula
Date posted:
March 26, 2019
.
Answers (1)
-
What factors should be considered when siting a plant to manufacture sulphuric acid?
(Solved)
What factors should be considered when siting a plant to manufacture sulphuric acid?
Date posted:
March 25, 2019
.
Answers (1)
-
List down different types of amorphous sulphide.
(Solved)
List down different types of amorphous sulphide.
Date posted:
March 25, 2019
.
Answers (1)
-
Why is it necessary to vulcanise natural rubber before use?
(Solved)
Why is it necessary to vulcanise natural rubber before use?
Date posted:
March 25, 2019
.
Answers (1)
-
Name one substance used for vulcanisation of rubber.
(Solved)
Name one substance used for vulcanisation of rubber.
Date posted:
March 25, 2019
.
Answers (1)
-
List four ammonium salts and state the uses of each.
(Solved)
List four ammonium salts and state the uses of each.
Date posted:
March 25, 2019
.
Answers (1)
-
Explain how carbon monoxide is lethal when one inhales.
(Solved)
Explain how carbon monoxide is lethal when one inhales.
Date posted:
March 25, 2019
.
Answers (1)
-
State six uses of carbon dioxide.
(Solved)
State six uses of carbon dioxide.
Date posted:
March 25, 2019
.
Answers (1)
-
How many molecules, by avogadro’s number does 0.32g of oxygen gas has?
(Solved)
How many molecules, by avogadro’s number does 0.32g of oxygen gas has?
Date posted:
March 25, 2019
.
Answers (1)
-
What mass of Lead (II) nitrate could contain 13g of Lead?
(Solved)
What mass of Lead (II) nitrate could contain 13g of Lead?
Date posted:
March 25, 2019
.
Answers (1)
-
How many calcium ions and chloride ions are in 1 mole of calcium chloride, CaCl2?.
(Solved)
How many calcium ions and chloride ions are in 1 mole of calcium chloride, CaCl2?.
Date posted:
March 25, 2019
.
Answers (1)
-
Calculate the number of nitrogen molecules in 560cm3 of nitrogen gas at s.t.p.
(Solved)
Calculate the number of nitrogen molecules in 560cm3 of nitrogen gas at s.t.p.
Date posted:
March 25, 2019
.
Answers (1)
-
How many grams of Sulphur contain 3.0 x 1021 atoms?.
(Solved)
How many grams of Sulphur contain 3.0 x 1021 atoms?.
Date posted:
March 25, 2019
.
Answers (1)
-
100cm3 of a gas at r.t.p was cooled to 60cm3. Calculate the new temperature of the gas in 0C if the pressure is kept constant.
(Solved)
100cm3 of a gas at r.t.p was cooled to 60cm3. Calculate the new temperature of the gas in 0C if the pressure is kept constant.
Date posted:
March 25, 2019
.
Answers (1)
-
Rates of diffusion of two gases A and B are in the ratio 2:1. If the molecular mass of gas A is 16g. Find the...
(Solved)
Rates of diffusion of two gases A and B are in the ratio 2:1. If the molecular mass of gas A is 16g. Find the molecular mass of B.
Date posted:
March 25, 2019
.
Answers (1)
-
In an experiment, it was found that a mole of a gas occupied 24.0dm3 at 200C and atmospheric pressure. What volume would it occupy at...
(Solved)
In an experiment, it was found that a mole of a gas occupied 24.0dm3 at 200C and atmospheric pressure. What volume would it occupy at S.T.P?
Date posted:
March 25, 2019
.
Answers (1)
-
Two gases, A and B have densities of 0.18gdm-3 and 2.90gdm-3 respectively. If they diffuse under the same conditions, what are their relative rates of...
(Solved)
Two gases, A and B have densities of 0.18gdm-3 and 2.90gdm-3 respectively. If they diffuse under the same conditions, what are their relative rates of diffusion.
Date posted:
March 25, 2019
.
Answers (1)
-
At 270C, nitrogen has a volume of 650cm3 under pressure of 980mmHg. What would be its volume at the same temperature but at a pressure...
(Solved)
At 270C, nitrogen has a volume of 650cm3 under pressure of 980mmHg. What would be its volume at the same temperature but at a pressure of 760mmHg?.
Date posted:
March 25, 2019
.
Answers (1)