Heat absorbed by ice from – 100 = 1x 2,100 x 10 = 2.1 x 104
Heat absorbed by melting ice= 1 x 334 x 104 = 3.34 x 105
Heat absorbed by water from 00 to 1000 = 1 x 4,200 x 100J = 4.2 x 106J
Heat absorbed water at 1000 = ML = 1 x 2,260 x 103 J= 2.26 x 106J.
Total heat absorbed = 301.5 x 104 = 3.035 x 106J
Heat given out by heater = power x time
Time = 3.035 x 106 =
6 x 103
= 0.5025 x 103
= 505 .83s = 8.43min
Wilfykil answered the question on March 26, 2019 at 13:43
-
An electric toy is rated 100W, 240V. Calculate the resistance of the toy when operating normally.
(Solved)
An electric toy is rated 100W, 240V. Calculate the resistance of the toy when operating normally.
Date posted:
March 26, 2019
.
Answers (1)
-
An electrical heater is labelled 120W, 240V. Calculate;
a) The current through the heating element when the heater is on.
b) The resistance of the element used...
(Solved)
An electrical heater is labelled 120W, 240V. Calculate;
a) The current through the heating element when the heater is on.
b) The resistance of the element used in the heater.
Date posted:
March 26, 2019
.
Answers (1)
-
An electrical appliance is rated as 240V, 200W. What does this information mean?
(Solved)
An electrical appliance is rated as 240V, 200W. What does this information mean?
Date posted:
March 26, 2019
.
Answers (1)
-
How many 100W electric irons could be safely connected to a 240V moving circuit fitted with a 13A fuse?
(Solved)
How many 100W electric irons could be safely connected to a 240V moving circuit fitted with a 13A fuse?
Date posted:
March 26, 2019
.
Answers (1)
-
When a current of 2A flows in a resistor for 10 minutes, 15KJ of electrical energy is dissipated. Determine the voltage across the resistor.
(Solved)
When a current of 2A flows in a resistor for 10 minutes, 15KJ of electrical energy is dissipated. Determine the voltage across the resistor.
Date posted:
March 26, 2019
.
Answers (1)
-
An electric bulb rated 40W is operating on 240V mains. Determine the resistance of its filament
(Solved)
An electric bulb rated 40W is operating on 240V mains. Determine the resistance of its filament
Date posted:
March 26, 2019
.
Answers (1)
-
One range of frequencies used in broadcasting varies from 0.5 x 100 Hz to 2.0 x 107 Hz. What is the longest wavelength of this...
(Solved)
One range of frequencies used in broadcasting varies from 0.5 x 100 Hz to 2.0 x 107 Hz. What is the longest wavelength of this range? Velocity of light air =3x108/s
Date posted:
March 26, 2019
.
Answers (1)
-
The figure below shows how the potential Energy (P.E) of a ball thrown vertically upwards.
(Solved)
The figure below shows how the potential Energy (P.E) of a ball thrown vertically upwards.

On the same axes, plot a graph of kinetic energy of the ball.
Date posted:
March 26, 2019
.
Answers (1)
-
The figure below shows a loaded wheelbarrow. Indicate and label on the diagram three forces acting on the wheelbarrow when a worker is just about...
(Solved)
The figure below shows a loaded wheelbarrow. Indicate and label on the diagram three forces acting on the wheelbarrow when a worker is just about to lift the handle.

Date posted:
March 26, 2019
.
Answers (1)
-
A body has 16 Joules of kinetic energy. What would be its kinetic energy if its velocity was double?
(Solved)
A body has 16 Joules of kinetic energy. What would be its kinetic energy if its velocity was double?
Date posted:
March 26, 2019
.
Answers (1)
-
A workshop has the following simple machines for lifting heavy loads, a wheel and axle, and a movable pulley. The wheel has a diameter of...
(Solved)
A workshop has the following simple machines for lifting heavy loads, a wheel and axle, and a movable pulley. The wheel has a diameter of 30cm while the axle has diameter of 3.0cm.
i) Sketch force diagrams to show how each machine works.
ii) Assuming that the machines are perfect. Calculate the mechanical advantage for each of the machines and show which machine is more advantageous in lifting loads.
Date posted:
March 26, 2019
.
Answers (1)
-
A car of mass 800 kg is initially moving at 25 m/s. Calculate the force needed to bring the car to rest over a distance...
(Solved)
A car of mass 800 kg is initially moving at 25 m/s. Calculate the force needed to bring the car to rest over a distance of 20 m.
Date posted:
March 26, 2019
.
Answers (1)
-
A ball rolls on a table in a straight line. A part from the transitional kinetic energy, state the other form of kinetic energy possessed...
(Solved)
A ball rolls on a table in a straight line. A part from the transitional kinetic energy, state the other form of kinetic energy possessed by the ball.
Date posted:
March 26, 2019
.
Answers (1)
-
A pump uses 1g of a mixture of petrol and alcohol in the ratio 4:1 by mass to raise 1000 kg of water from a...
(Solved)
A pump uses 1g of a mixture of petrol and alcohol in the ratio 4:1 by mass to raise 1000 kg of water from a well 200m deep.
i) How much energy is given by 1g of mixture?
ii) If the pump is 40% efficient, what mass of this mixture is needed to raise the water? (1g of alcohol = 7000J, of petrol= 48000J)
Date posted:
March 26, 2019
.
Answers (1)
-
An energy saving stove when burning steadily has an efficiency of 69%. The stove melts 0.03 kg lf ice 00C in 180 seconds.
(Solved)
An energy saving stove when burning steadily has an efficiency of 69%. The stove melts 0.03 kg lf ice 00C in 180 seconds.
Calculate: -
i) The power rating of the stove.
ii) The heat energy wasted by the stove.
Date posted:
March 26, 2019
.
Answers (1)
-
Figure below shows a block of mass 30.0 kg being pulled up a slope by force P at a constant speed. The frictional force on...
(Solved)
Figure below shows a block of mass 30.0 kg being pulled up a slope by force P at a constant speed. The frictional force on the block is 20.0N
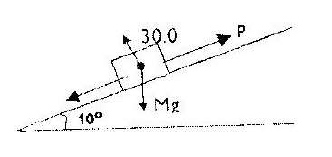
i) On the same figure name and indicate other forces acting on the block.
ii) Determine the component of the weight acting on the trolley down the slope
iii) Determine the value of P.
Date posted:
March 26, 2019
.
Answers (1)
-
The fig. shows a 2 kg block attached to 0.5 kg mass by a light in-extensible string which passes over a pulley. The force of...
(Solved)
The fig. shows a 2 kg block attached to 0.5 kg mass by a light in-extensible string which passes over a pulley. The force of friction between the horizontal bench and block is 3N. The block is released from rest so that both masses move through a distance of 0.6m. Calculate the velocity of the string.
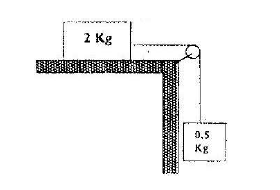
Date posted:
March 26, 2019
.
Answers (1)
-
In figure shown below. Two identical electroscopes A and B carry the same type of charges as shown. The two are then connected with a...
(Solved)
In figure shown below. Two identical electroscopes A and B carry the same type of charges as shown. The two are then connected with a copper wire.
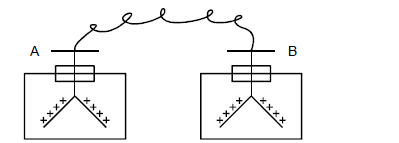
State and explain the observations made.
Date posted:
March 26, 2019
.
Answers (1)
-
A body of mass 5 kg is ejected vertically from the ground when a force of 600N acts on it for 0.1s. Calculate the velocity...
(Solved)
A body of mass 5 kg is ejected vertically from the ground when a force of 600N acts on it for 0.1s. Calculate the velocity with which the body leaves the ground.
Date posted:
March 26, 2019
.
Answers (1)
-
Two coins were placed at the bottom of two jars each containing a different clear liquid as shown in figure below.
(Solved)
Two coins were placed at the bottom of two jars each containing a different clear liquid as shown in figure below.
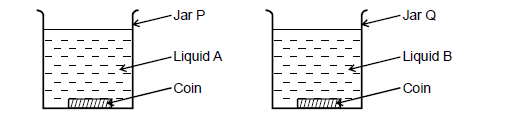
The liquids in the two jars are at the same level. The coin in jar q appears shallower than that in jar P. Explain.
Date posted:
March 26, 2019
.
Answers (1)