- Use the set up below to answer the questions that follow.(Solved)
Use the set up below to answer the questions that follow.
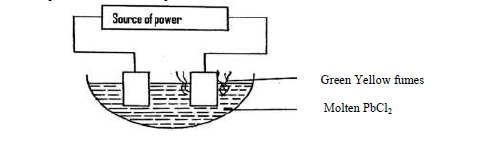
(a) On the diagram, label the cathode.
(b) Write the equation for the reaction on the cathode.
Date posted: March 27, 2019. Answers (1)
- Study the flow chart below and answer the questions that follow.(Solved)
Study the flow chart below and answer the questions that follow.
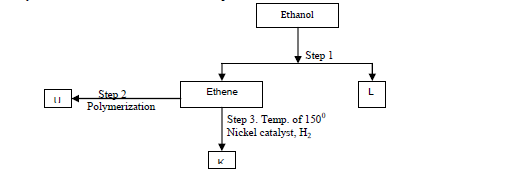
(a) Identify substances: K, U,L
(b) State the conditions for the reaction in step 1 to occur.
(c) Give one disadvantage of continued use of substances such as U.
Date posted: March 27, 2019. Answers (1)
- (a) Define the term oxidation state.
(b) Calculate the oxidation states of chromium and manganese in the following ions.
(i) Chromium in Cr2O72-
(ii) Manganese in...(Solved)
(a) Define the term oxidation state.
(b) Calculate the oxidation states of chromium and manganese in the following ions.
(i) Chromium in Cr2O72-
(ii) Manganese in MnO4-
Date posted: March 27, 2019. Answers (1)
- In an experiment to study properties of carbon, a small amount of charcoal is placed in a boiling tube. 5.0cm3 of concentrated nitric acid is...(Solved)
In an experiment to study properties of carbon, a small amount of charcoal is placed in a boiling tube. 5.0cm3 of concentrated nitric acid is added. The mixture is then heated.
(a) What observations are made?
(b) Write an equation for the reaction that took place in the boiling tube.
(c) What property of carbon is shown in this reaction?
Date posted: March 27, 2019. Answers (1)
- Study the flow chart below and answer the questions that follow.(Solved)
Study the flow chart below and answer the questions that follow.

(a) Identify solid R.
(b) Write a balanced equation for step II and ionic equation for step III.
Step II
Step III
Date posted: March 27, 2019. Answers (1)
- An organic compound contains carbon and hydrogen only. When this compound was completely burnt in excess air, it gave 9.6g of Carbon (IV) Oxide and...(Solved)
An organic compound contains carbon and hydrogen only. When this compound was completely burnt in excess air, it gave 9.6g of Carbon (IV) Oxide and 4.9g of water vapour. The molecular mass of the hydrocarbon is 58. Determine the molecular formula. (C = 12, O = 16, H = 1)
Date posted: March 27, 2019. Answers (1)
- 200cm3 of Nitrogen (I) Oxide (N2O) pass through a porous plug in 2 minute 15 seconds. How long will it take the same volume of...(Solved)
200cm3 of Nitrogen (I) Oxide (N2O) pass through a porous plug in 2 minute 15 seconds. How long will it take the same volume of Sulphur (IV) Oxide (SO2) gas to diffuse through the same plug under the same conditions? (N = 14, O = 16, S = 32)
Date posted: March 27, 2019. Answers (1)
- The graph below shows the amount of calcium carbonate and calcium chloride varying with time in the reaction.(Solved)
The graph below shows the amount of calcium carbonate and calcium chloride varying with time in the reaction.
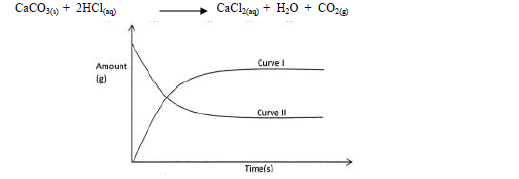
(a) Which curve shows the amount of calcium chloride varying with time?
(b) Explain why the two curves become horizontal after a given period of time.
Date posted: March 27, 2019. Answers (1)
- The table below gives the solubility of potassium bromide and potassium sulphate at 00C and 400C.(Solved)
The table below gives the solubility of potassium bromide and potassium sulphate at 00C and 400C.

When an aqueous mixture containing 60g of potassium bromide and 7g potassium sulphate in 100g of water at 800C was cooled to 00C, some crystals were formed.
(a) Identify the crystals.
(b) Determine the mass of the crystals.
Date posted: March 27, 2019. Answers (1)
- Study the figure below and answer questions that follow.(Solved)
Study the figure below and answer questions that follow.
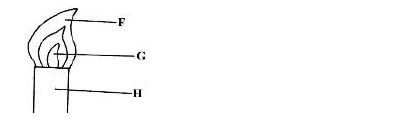
Name the parts labelled F and G.
Date posted: March 27, 2019. Answers (1)
- Study the diagram below and use it to answer the questions that follow.(Solved)
Study the diagram below and use it to answer the questions that follow.
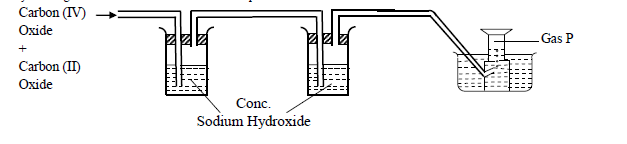
(a) Name two reagents that are reacted to produce both Carbon (IV) Oxide and Carbon (II) Oxide.
(b) Write the equation for the reactions that took place in the wash bottle.
(c) Give a reason why Carbon (II) Oxide is not easily detected.
Date posted: March 27, 2019. Answers (1)
- The structure below represents two cleansing agents A and B. Which cleansing agent would be suitable for washing in water containing calcium chloride? Give a...(Solved)
The structure below represents two cleansing agents A and B. Which cleansing agent would be suitable for washing in water containing calcium chloride? Give a reason.

Date posted: March 26, 2019. Answers (1)
- A student put calcium carbonate and calcium hydrogen carbonate in separate test tubes and performed
the tests as shown in the table below. Complete the table...(Solved)
A student put calcium carbonate and calcium hydrogen carbonate in separate test tubes and performed
the tests as shown in the table below. Complete the table by giving the expected observations.

Date posted: March 26, 2019. Answers (1)
- Study the scheme below and answer the questions that follow.(Solved)
Study the scheme below and answer the questions that follow.
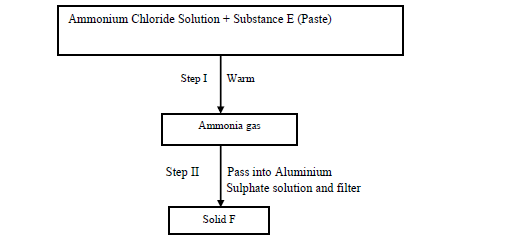
(a) Identify substance E
(b) Write an equation for the reaction in Step (II) that produces solid F
Date posted: March 26, 2019. Answers (1)
- The products of a burning candle were passed through a tube containing calcium oxide as shown in the diagram below.(Solved)
The products of a burning candle were passed through a tube containing calcium oxide as shown in the diagram below.
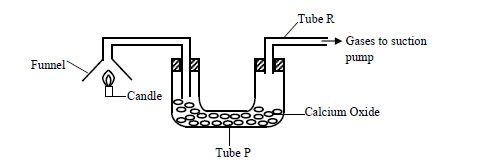
(a) Write two chemical equations for the reactions that took place in tube P.
(b) Name two gases that came out through tube R.
Date posted: March 26, 2019. Answers (1)
- Excess Carbon (II) Oxide was passed over a heated sample of an oxide of iron as shown in the diagram below. Study the diagram and...(Solved)
Excess Carbon (II) Oxide was passed over a heated sample of an oxide of iron as shown in the diagram below. Study the diagram and the data and use it to answer the questions that follow
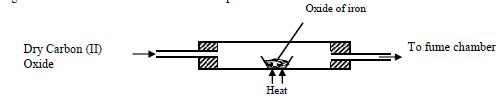
Mass of empty dish =6.72g
Mass of empty dish + oxide of iron =9.04g
Mass of empty dish + residue=8.40g
(a) Determine the formula of the oxide of iron given that the relative formula mass of oxide of Iron = 232, Fe = 56.0, O=16.0
(b) Write an equation for the reaction which took place in the dish
Date posted: March 26, 2019. Answers (1)
- The elements A and B have the following properties(Solved)
The elements A and B have the following properties

(a) When the isotope A was bombarded with a neutron, an isotope C was formed .Fill in the table to show the properties of element C
(b)Write an equation for the reaction between isotope B and Beta particles
(c) State one use of radioisotopes in medicine.
Date posted: March 26, 2019. Answers (1)
- The set up below was used to electrolyze molten lead (II) bromide.(Solved)
The set up below was used to electrolyze molten lead (II) bromide.
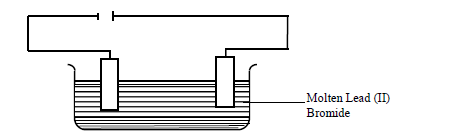
(a) State the observation that was made at the anode during electrolysis.
(b) A current of 2.5A was passed for 30 minutes. Calculate the mass of lead that was deposited
Date posted: March 26, 2019. Answers (1)
- Use the information below and answer the questions that follow .The letters are not the actual symbols of the elements.(Solved)
Use the information below and answer the questions that follow .The letters are not the actual symbols of the elements.
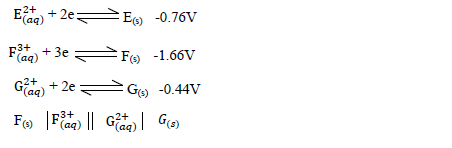
(a) Calculate the E? value for the electrochemical cell represented below.
(b) Arrange the elements in order of reactivity starting with the least reactive.
(c) Explain if it would be advisable to store element G in a solution containing E2+ Ions.
Date posted: March 26, 2019. Answers (1)
- The table below gives some properties of three elements X,Y and Z.(Solved)
The table below gives some properties of three elements X,Y and Z.
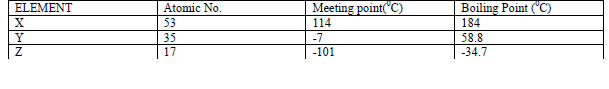
(a) Which element is in liquid form at room temperature? Give reason.
(b) Explain why the boiling point of element X is higher than that of element Z.
Date posted: March 26, 2019. Answers (1)