(a) Nitrogen(IV) oxide(NO2) gas
(b) (i) 4NH3(g) +5O2(g)---->4NO(g) + 6H2O(g)
(ii) 2NO(g) + O2(g)---->2NO2(g)
sharon kalunda answered the question on March 27, 2019 at 07:33
- State three reasons why air is considered to be a mixture but not a compound.(Solved)
State three reasons why air is considered to be a mixture but not a compound.
Date posted: March 27, 2019. Answers (1)
- Below is a set-up of apparatus used to prepare hydrogen gas in the laboratory. Study it and answer the questions that follow.(Solved)
Below is a set-up of apparatus used to prepare hydrogen gas in the laboratory. Study it and answer the questions that follow.

(a) Write a chemical equation for the two reactions taking place in he above set-up.
(b) State the chemical test for hydrogen gas.
Date posted: March 27, 2019. Answers (1)
- In an experiment, concentrated sulphuric (VI) acid was put in a beaker and exposed to air for one week as shown below.(Solved)
In an experiment, concentrated sulphuric (VI) acid was put in a beaker and exposed to air for one week as shown below.

(i) What observation was made after one week? Explain.
(ii) What property of sulphuric (VI) acid was being investigated in the experiment?
Date posted: March 27, 2019. Answers (1)
- Use the bond energy value given below for the question that follows.
Bond ...(Solved)
Use the bond energy value given below for the question that follows.
Bond Bond energy (kJmol-1)
H – H 432
C = C 610
C – C 346
C – H 413
Determine the enthalpy change for the conversion of butene to butane by hydrogen.
Date posted: March 27, 2019. Answers (1)
- Use the set up below to answer the questions that follow.(Solved)
Use the set up below to answer the questions that follow.
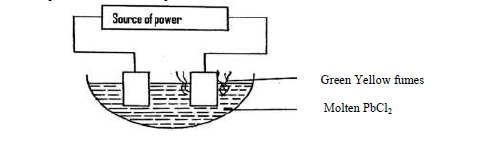
(a) On the diagram, label the cathode.
(b) Write the equation for the reaction on the cathode.
Date posted: March 27, 2019. Answers (1)
- Study the flow chart below and answer the questions that follow.(Solved)
Study the flow chart below and answer the questions that follow.
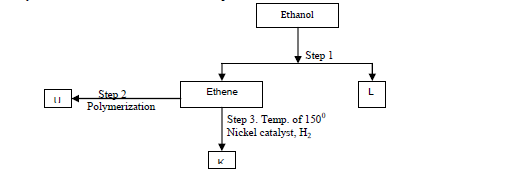
(a) Identify substances: K, U,L
(b) State the conditions for the reaction in step 1 to occur.
(c) Give one disadvantage of continued use of substances such as U.
Date posted: March 27, 2019. Answers (1)
- (a) Define the term oxidation state.
(b) Calculate the oxidation states of chromium and manganese in the following ions.
(i) Chromium in Cr2O72-
(ii) Manganese in...(Solved)
(a) Define the term oxidation state.
(b) Calculate the oxidation states of chromium and manganese in the following ions.
(i) Chromium in Cr2O72-
(ii) Manganese in MnO4-
Date posted: March 27, 2019. Answers (1)
- In an experiment to study properties of carbon, a small amount of charcoal is placed in a boiling tube. 5.0cm3 of concentrated nitric acid is...(Solved)
In an experiment to study properties of carbon, a small amount of charcoal is placed in a boiling tube. 5.0cm3 of concentrated nitric acid is added. The mixture is then heated.
(a) What observations are made?
(b) Write an equation for the reaction that took place in the boiling tube.
(c) What property of carbon is shown in this reaction?
Date posted: March 27, 2019. Answers (1)
- Study the flow chart below and answer the questions that follow.(Solved)
Study the flow chart below and answer the questions that follow.

(a) Identify solid R.
(b) Write a balanced equation for step II and ionic equation for step III.
Step II
Step III
Date posted: March 27, 2019. Answers (1)
- An organic compound contains carbon and hydrogen only. When this compound was completely burnt in excess air, it gave 9.6g of Carbon (IV) Oxide and...(Solved)
An organic compound contains carbon and hydrogen only. When this compound was completely burnt in excess air, it gave 9.6g of Carbon (IV) Oxide and 4.9g of water vapour. The molecular mass of the hydrocarbon is 58. Determine the molecular formula. (C = 12, O = 16, H = 1)
Date posted: March 27, 2019. Answers (1)
- 200cm3 of Nitrogen (I) Oxide (N2O) pass through a porous plug in 2 minute 15 seconds. How long will it take the same volume of...(Solved)
200cm3 of Nitrogen (I) Oxide (N2O) pass through a porous plug in 2 minute 15 seconds. How long will it take the same volume of Sulphur (IV) Oxide (SO2) gas to diffuse through the same plug under the same conditions? (N = 14, O = 16, S = 32)
Date posted: March 27, 2019. Answers (1)
- The graph below shows the amount of calcium carbonate and calcium chloride varying with time in the reaction.(Solved)
The graph below shows the amount of calcium carbonate and calcium chloride varying with time in the reaction.
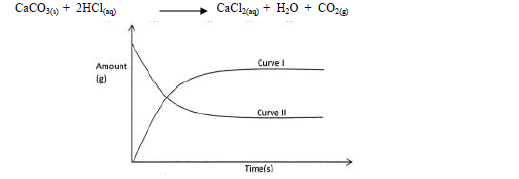
(a) Which curve shows the amount of calcium chloride varying with time?
(b) Explain why the two curves become horizontal after a given period of time.
Date posted: March 27, 2019. Answers (1)
- The table below gives the solubility of potassium bromide and potassium sulphate at 00C and 400C.(Solved)
The table below gives the solubility of potassium bromide and potassium sulphate at 00C and 400C.

When an aqueous mixture containing 60g of potassium bromide and 7g potassium sulphate in 100g of water at 800C was cooled to 00C, some crystals were formed.
(a) Identify the crystals.
(b) Determine the mass of the crystals.
Date posted: March 27, 2019. Answers (1)
- Study the figure below and answer questions that follow.(Solved)
Study the figure below and answer questions that follow.
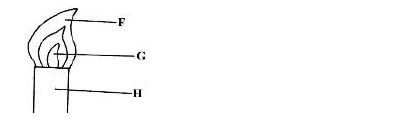
Name the parts labelled F and G.
Date posted: March 27, 2019. Answers (1)
- Study the diagram below and use it to answer the questions that follow.(Solved)
Study the diagram below and use it to answer the questions that follow.
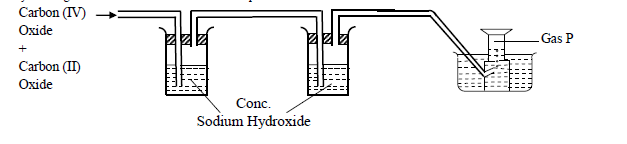
(a) Name two reagents that are reacted to produce both Carbon (IV) Oxide and Carbon (II) Oxide.
(b) Write the equation for the reactions that took place in the wash bottle.
(c) Give a reason why Carbon (II) Oxide is not easily detected.
Date posted: March 27, 2019. Answers (1)
- The structure below represents two cleansing agents A and B. Which cleansing agent would be suitable for washing in water containing calcium chloride? Give a...(Solved)
The structure below represents two cleansing agents A and B. Which cleansing agent would be suitable for washing in water containing calcium chloride? Give a reason.

Date posted: March 26, 2019. Answers (1)
- A student put calcium carbonate and calcium hydrogen carbonate in separate test tubes and performed
the tests as shown in the table below. Complete the table...(Solved)
A student put calcium carbonate and calcium hydrogen carbonate in separate test tubes and performed
the tests as shown in the table below. Complete the table by giving the expected observations.

Date posted: March 26, 2019. Answers (1)
- Study the scheme below and answer the questions that follow.(Solved)
Study the scheme below and answer the questions that follow.
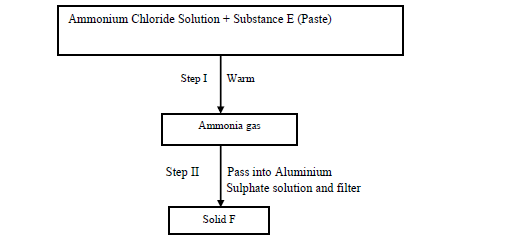
(a) Identify substance E
(b) Write an equation for the reaction in Step (II) that produces solid F
Date posted: March 26, 2019. Answers (1)
- The products of a burning candle were passed through a tube containing calcium oxide as shown in the diagram below.(Solved)
The products of a burning candle were passed through a tube containing calcium oxide as shown in the diagram below.
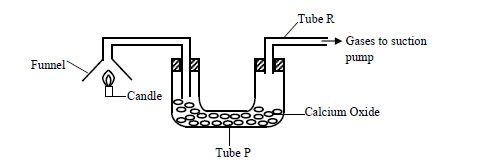
(a) Write two chemical equations for the reactions that took place in tube P.
(b) Name two gases that came out through tube R.
Date posted: March 26, 2019. Answers (1)
- Excess Carbon (II) Oxide was passed over a heated sample of an oxide of iron as shown in the diagram below. Study the diagram and...(Solved)
Excess Carbon (II) Oxide was passed over a heated sample of an oxide of iron as shown in the diagram below. Study the diagram and the data and use it to answer the questions that follow
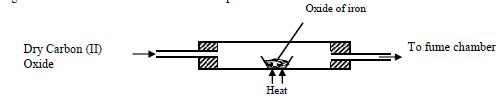
Mass of empty dish =6.72g
Mass of empty dish + oxide of iron =9.04g
Mass of empty dish + residue=8.40g
(a) Determine the formula of the oxide of iron given that the relative formula mass of oxide of Iron = 232, Fe = 56.0, O=16.0
(b) Write an equation for the reaction which took place in the dish
Date posted: March 26, 2019. Answers (1)