Ultra violet has a higher energy than yellow light.
E = hf = hc
Wilfykil answered the question on March 27, 2019 at 08:20
- Distinguish between a photon and a quantum.(Solved)
Distinguish between a photon and a quantum.
Date posted: March 27, 2019. Answers (1)
- Give one important use of each of the following em-waves.
i) Microwaves
ii) Infrared(Solved)
Give one important use of each of the following em-waves.
i) Microwaves
ii) Infrared
Date posted: March 27, 2019. Answers (1)
- State where Gamma rays originate.(Solved)
State where Gamma rays originate.
Date posted: March 27, 2019. Answers (1)
- State the origin of all electromagnetic-radiation from radio waves to x-rays(Solved)
State the origin of all electromagnetic-radiation from radio waves to x-rays
Date posted: March 27, 2019. Answers (1)
- Name two properties of ultraviolet radiation.(Solved)
Name two properties of ultraviolet radiation.
Date posted: March 27, 2019. Answers (1)
- State 3 uses of infrared radiation.(Solved)
State 3 uses of infrared radiation.
Date posted: March 27, 2019. Answers (1)
- Other than a photographic film state one other detectors of:
(Solved)
Other than a photographic film state one other detectors of:
i) X-rays
ii) UV,
iii) Visible spectrum
iv) Infra-red radiations
Date posted: March 27, 2019. Answers (1)
- Name two types of electromagnetic radiations whose frequencies are greater than that of visible light.(Solved)
Name two types of electromagnetic radiations whose frequencies are greater than that of visible light.
Date posted: March 27, 2019. Answers (1)
- Arrange the following radiations in order of increasing wavelengths.(Solved)
Arrange the following radiations in order of increasing wavelengths.
Ultraviolet, Gamma Rays, Radio Waves, Infra Red
Date posted: March 27, 2019. Answers (1)
- State one-way of detecting ultra violet radiation(Solved)
State one-way of detecting ultra violet radiation
Date posted: March 27, 2019. Answers (1)
- A uniform plank of wood is pivoted at its centre. A block of wood of mass 2kg is balanced by a mass of 1.5 placed...(Solved)
A uniform plank of wood is pivoted at its centre. A block of wood of mass 2kg is balanced by a mass of 1.5 placed 30cm from the pivot as shown.
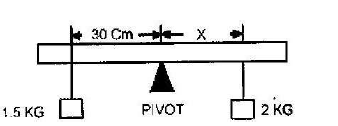
i) Calculate the distance X
ii) When the same block of wood is partially immersed in water, the 1.5kg mass need to be placed at 20cm from the pivot to balance it. Calculate the weight of the water displaced.
Date posted: March 27, 2019. Answers (1)
- A right angled solid of dimensions 0.02m by 0.02m by 0.2m and density 2,700kg/m3 is supported inside kerosene of density 800kg/m3 by a thread which...(Solved)
A right angled solid of dimensions 0.02m by 0.02m by 0.2m and density 2,700kg/m3 is supported inside kerosene of density 800kg/m3 by a thread which is attached to a spring balance. The long side is vertical and the upper surface is 0.1m below the surface of the kerosene.
i) Calculate the force due to the liquid on the lower upper surface of the solid.
ii) Calculate the up thrust and determine the reading on the spring balance.
Date posted: March 27, 2019. Answers (1)
- The figure below shows a cube of a certain wood whose density is the same as that of water. The cube is held on the...(Solved)
The figure below shows a cube of a certain wood whose density is the same as that of water. The cube is held on the surface of the water in a long cylinder. Explain what happens to the cube after it is released
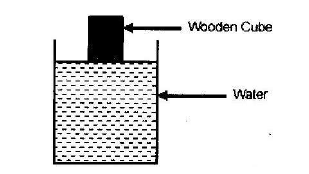
Date posted: March 27, 2019. Answers (1)
- A mass of 120 g half immersed in water displaced a volume of 20 cm3. Calculate the density of the object(Solved)
A mass of 120 g half immersed in water displaced a volume of 20 cm3. Calculate the density of the object
Date posted: March 27, 2019. Answers (1)
- Determine the density of glass that weighs 0.5N in air and 0.3N in water.(Solved)
Determine the density of glass that weighs 0.5N in air and 0.3N in water.
Date posted: March 27, 2019. Answers (1)
- State how a hydrometer may be used to test whether a car battery is fully charged.(Solved)
State how a hydrometer may be used to test whether a car battery is fully charged.
Date posted: March 27, 2019. Answers (1)
- A child of mass 20kg sits on a swing of length 4m and swings through a vertical height of 0.9m as shown in the figure...(Solved)
A child of mass 20kg sits on a swing of length 4m and swings through a vertical height of 0.9m as shown in the figure below.
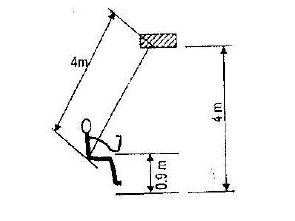
Determine the:
i) Speed of the child when passing through the lowest point.
ii) Force exerted on the child by the seat of the swing when passing through the lowest point.
Date posted: March 27, 2019. Answers (1)
- A body moving with uniform angular velocity found to have covered an angular distance 170 radians in t seconds. Thirteen seconds later it is found...(Solved)
A body moving with uniform angular velocity found to have covered an angular distance 170 radians in t seconds. Thirteen seconds later it is found to have covered a total angular distance of 300 radians. Determine t
Date posted: March 27, 2019. Answers (1)
- A small object moving in a horizontal circle of radius 0.2m makes 8 revolutions per second. Determine its centripetal acceleration.(Solved)
A small object moving in a horizontal circle of radius 0.2m makes 8 revolutions per second. Determine its centripetal acceleration.
Date posted: March 27, 2019. Answers (1)
- The rear wheel of a certain car has a diameter of 40cm. At a certain speed of the car, the wheel makes 7 revolutions per...(Solved)
The rear wheel of a certain car has a diameter of 40cm. At a certain speed of the car, the wheel makes 7 revolutions per second. A small stone embedded in the tyre tread flies off initially at an angle of 450 to the ground. Determine the initial velocity of the pebble(take pi = 22/7)
Date posted: March 27, 2019. Answers (1)