-
A radio repairer wishes to use an ammeter to detect a faulty diode. With the aid of a circuit diagram describe how he will go...
(Solved)
A radio repairer wishes to use an ammeter to detect a faulty diode. With the aid of a circuit diagram describe how he will go about this task.
Date posted:
March 27, 2019
.
Answers (1)
-
Name the quantities, which must be measured so as to determine the half-life of a radioactive sample whose half-life is known to be a few...
(Solved)
Name the quantities, which must be measured so as to determine the half-life of a radioactive sample whose half-life is known to be a few hours.
Date posted:
March 27, 2019
.
Answers (1)
-
State two factors that determine the extent of the damage to the body cell caused by the radiation from radioactive substances.
(Solved)
State two factors that determine the extent of the damage to the body cell caused by the radiation from radioactive substances.
Date posted:
March 27, 2019
.
Answers (1)
-
A monochromatic beam of radiation is directed on a clean metal surface so as to produce photo-electrons. Give a reason why some of the ejected...
(Solved)
A monochromatic beam of radiation is directed on a clean metal surface so as to produce photo-electrons. Give a reason why some of the ejected photo-electrons have more kinetic energy than others.
Date posted:
March 27, 2019
.
Answers (1)
-
The diagram shows a photocell in action
(Solved)
The diagram shows a photocell in action
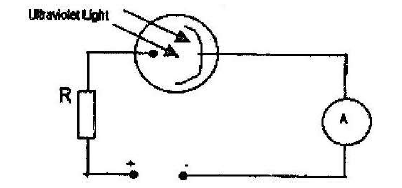
i) The photocell is either evacuated or filled with an inert gas at low pressure. Give one reason for this
ii) What is the function of the resistor R in the circuit?
iii) State one reason for using a particular radiation such as ultraviolet for a given photocell.
iv) Explain how the set-up shown in the diagram may be used as an automatic switching device for a burglar alarm.
Date posted:
March 27, 2019
.
Answers (1)
-
Light of frequency 5.5x 1014 HZ is made to strike a surface whose work function is 2.5ev. Show that photoelectric effect will not take place....
(Solved)
Light of frequency 5.5x 1014 HZ is made to strike a surface whose work function is 2.5ev. Show that photoelectric effect will not take place. H= 6.6 X 1034Js
Date posted:
March 27, 2019
.
Answers (1)
-
State the purpose of cooling fins in the X-ray tube.
(Solved)
State the purpose of cooling fins in the X-ray tube.
Date posted:
March 27, 2019
.
Answers (1)
-
Explain why X-rays are appropriate in study of the crystalline structure materials.
(Solved)
Explain why X-rays are appropriate in study of the crystalline structure materials.
Date posted:
March 27, 2019
.
Answers (1)
-
An accelerating potential of 20kv is applied to an X-ray tube.
(Solved)
An accelerating potential of 20kv is applied to an X-ray tube.
i) What is the velocity with which the electron strikes the target?
ii) State the energy changes that take place at the target.
Date posted:
March 27, 2019
.
Answers (1)
-
For a given source of X-rays, how would the following be controlled.
(Solved)
For a given source of X-rays, how would the following be controlled.
i) Intensity
ii) The penetrating power
iii) The exposure to patients
Date posted:
March 27, 2019
.
Answers (1)
-
A photon has an energy of 5x10-19J. Calculate the wavelength associated with this photon.
(Solved)
A photon has an energy of 5x10-19J. Calculate the wavelength associated with this photon.
Date posted:
March 27, 2019
.
Answers (1)
-
How does the energy of ultra violet light compare to that of yellow light given that the energy E of a wave frequency f, is...
(Solved)
How does the energy of ultra violet light compare to that of yellow light given that the energy E of a wave frequency f, is given by E = hf, where h is plank’s constant?
Date posted:
March 27, 2019
.
Answers (1)
-
Distinguish between a photon and a quantum.
(Solved)
Distinguish between a photon and a quantum.
Date posted:
March 27, 2019
.
Answers (1)
-
State where Gamma rays originate.
(Solved)
State where Gamma rays originate.
Date posted:
March 27, 2019
.
Answers (1)
-
State the origin of all electromagnetic-radiation from radio waves to x-rays
(Solved)
State the origin of all electromagnetic-radiation from radio waves to x-rays
Date posted:
March 27, 2019
.
Answers (1)
-
Name two properties of ultraviolet radiation.
(Solved)
Name two properties of ultraviolet radiation.
Date posted:
March 27, 2019
.
Answers (1)
-
State 3 uses of infrared radiation.
(Solved)
State 3 uses of infrared radiation.
Date posted:
March 27, 2019
.
Answers (1)
-
Name two types of electromagnetic radiations whose frequencies are greater than that of visible light.
(Solved)
Name two types of electromagnetic radiations whose frequencies are greater than that of visible light.
Date posted:
March 27, 2019
.
Answers (1)
-
Arrange the following radiations in order of increasing wavelengths.
(Solved)
Arrange the following radiations in order of increasing wavelengths.
Ultraviolet, Gamma Rays, Radio Waves, Infra Red
Date posted:
March 27, 2019
.
Answers (1)
-
State one-way of detecting ultra violet radiation
(Solved)
State one-way of detecting ultra violet radiation
Date posted:
March 27, 2019
.
Answers (1)