- 100cm3 of methane gas diffused through a porous partition in 40 seconds. How long would it take 90cm3 of ozone gas to diffuse through the...(Solved)
100cm3 of methane gas diffused through a porous partition in 40 seconds. How long would it take 90cm3 of ozone gas to diffuse through the same partition? (C = 12, H = 1, O = 16)
Date posted: March 27, 2019. Answers (1)
- The diagram below shows part of Solvay Process.(Solved)
The diagram below shows part of Solvay Process.

(a) Name solid P
(b) State the process taking place in chamber N.
(c) State two uses of calcium chloride which is a by-product in this process.
Date posted: March 27, 2019. Answers (1)
- The diagram below shows a set up which was used by student to investigate effect of electricity on solid Molten Lead (II) Bromide. Study it...(Solved)
The diagram below shows a set up which was used by student to investigate effect of electricity on solid Molten Lead (II) Bromide. Study it and answer the questions that follow.

(a) (i) State and explain the observation at the anode when the switch is switched on.
(ii) What precaution should be taken when carrying out this experiment?
(iii) Write the equation of the reaction taking place at the Anode.
(iv) Why are graphite electrodes used in the experiment?
(v) On the diagram, indicate the direction of flow of electrons.
(vi) The students noted that the bulb only produced light after the Lead (II) Bromide had melted. Explain this observation.
(b) State the difference in conduction of electric current between Molten Lead (II) Bromide and Lead Metal.
(c) Explain why it is not advisable to store Copper (II) Sulphate solution in a can made of Zinc metal.
(d) State two applications of electrolysis.
Date posted: March 27, 2019. Answers (1)
- The flow chart below shows the Haber process in the large scale manufacture of Ammonia gas. Use it to answer the questions that follow.(Solved)
The flow chart below shows the Haber process in the large scale manufacture of Ammonia gas. Use it to answer the questions that follow.

Describe how nitrogen is obtained from air on a large scale.
(a) (i) Name one source of hydrogen gas used as a raw material in the above process.
(ii) Name chamber A.
(iii) Write an equation for the reaction taking place in the catalytic chamber.
(iv) In the Haber process optimum temperature of 5000C and 200 atmospheres of pressure are used to get optimum yield of
Ammonia. Why can’t lower temperatures and higher pressure be used?
(b) Give two reasons why finely divided iron is the commonly used catalyst.
(c) State and explain the observation made when dry ammonia gas is passed over heated copper (II) Oxide
in a combustion tube.
(d) Give two uses of ammonia gas.
Date posted: March 27, 2019. Answers (1)
- Study the flow chart below and answer the questions that follow.(Solved)
Study the flow chart below and answer the questions that follow.

(i) Name the process taking place in step (I).
(ii) Describe chemical test that can be carried out to show the identity of organic compound A.
(iii) Give the name of the following:
I. A:
II. B:
(iv) Give the structural formulae of substance C.
(v) Name the type of reaction that occurs in:
I. Step IV
II. Step VI:
(vi) Give the reagent and the condition necessary for step VI.
Reagent:
Condition:
(b) Give the systematic names of the following compounds:
I. CH2CHCHCH2CH3
II. CH C C H3
Date posted: March 27, 2019. Answers (1)
- During Olympics, urine sample of five short distance runners were taken and tested for the presence of two illegal steroids by paper chromatography. Methanol was...(Solved)
During Olympics, urine sample of five short distance runners were taken and tested for the presence of two illegal steroids by paper chromatography. Methanol was used as the solvent. A chromatogram from the test appeared as shown below. Study the chromatogram and answer the questions that follow.

(a) Which of the two steroids is most likely to be more soluble in methanol? Give a reason.
(b) Identify the athletes that tested positive for the illegal steroids.
Date posted: March 27, 2019. Answers (1)
- Some salts may be classified as double salts or basic salts. Trona with the formula Na2CO3.NaHCO3 is an example of a double salt. An example...(Solved)
Some salts may be classified as double salts or basic salts. Trona with the formula Na2CO3.NaHCO3 is an example of a double salt. An example of a basic salt is basic magnesium carbonate with formula MgCO3.Mg(OH)2.
(a) What is meant by a double salt?
(b) Write equations of reactions that occur when dilute hydrochloric acid is reacted with:
(i) Trona
(ii) Basic magnesium carbonate.
Date posted: March 27, 2019. Answers (1)
- Using dots (•) and crosses (x) to represent electrons, show bonding in the compound formed when the following elements
reacts. (N = 14, H = 1).
Nitrogen...(Solved)
Using dots (•) and crosses (x) to represent electrons, show bonding in the compound formed when the following elements reacts. (N = 14, H = 1).
Nitrogen and Hydrogen.
Date posted: March 27, 2019. Answers (1)
- The table below shows the pH values of some solutions.(Solved)
The table below shows the pH values of some solutions.

(a) Which solution is likely to be:
(i) Potassium hydroxide
(ii) Lemon juice
(b) Explain why a solution of hydrogen chloride gas in methyl benzene was identified as N.
Date posted: March 27, 2019. Answers (1)
- A gaseous compound consists of 86% carbon and 14% hydrogen by mass. At s.t.p. 3.2dm3 of the compound had a mass of 6g. Calculate its...(Solved)
A gaseous compound consists of 86% carbon and 14% hydrogen by mass. At s.t.p. 3.2dm3 of the compound had a mass of 6g. Calculate its molecular formula.(1 mole of a gas at s.t.p. = 22.4dm3)
Date posted: March 27, 2019. Answers (1)
- The 1st, 2nd and 3rd ionization energies in KJ/Mol of elements G and R are given below.(Solved)
The 1st, 2nd and 3rd ionization energies in KJ/Mol of elements G and R are given below.

(i) Define the term 1st ionization energy.
(ii) Apart from the decrease in energy levels, explain the big difference between the 1st and 2nd ionization energies.
(iii) Calculate the amount of energy for the process:

Date posted: March 27, 2019. Answers (1)
- An experiment was set up using chlorine water as shown below.(Solved)
An experiment was set up using chlorine water as shown below.

(i) Identify gas X.
(ii) Write an equation for the production of gas X.
Date posted: March 27, 2019. Answers (1)
- The set-up below shows the catalytic oxidation of ammonia in the laboratory.(Solved)
The set-up below shows the catalytic oxidation of ammonia in the laboratory.

(a) Identify the brown fumes observed at the mouth of the conical flask.
(b) Write down the equations of the reactions representing
(i) Catalytic oxidation of ammonia
(ii) The formation of the brown fumes.
Date posted: March 27, 2019. Answers (1)
- State three reasons why air is considered to be a mixture but not a compound.(Solved)
State three reasons why air is considered to be a mixture but not a compound.
Date posted: March 27, 2019. Answers (1)
- Below is a set-up of apparatus used to prepare hydrogen gas in the laboratory. Study it and answer the questions that follow.(Solved)
Below is a set-up of apparatus used to prepare hydrogen gas in the laboratory. Study it and answer the questions that follow.

(a) Write a chemical equation for the two reactions taking place in he above set-up.
(b) State the chemical test for hydrogen gas.
Date posted: March 27, 2019. Answers (1)
- In an experiment, concentrated sulphuric (VI) acid was put in a beaker and exposed to air for one week as shown below.(Solved)
In an experiment, concentrated sulphuric (VI) acid was put in a beaker and exposed to air for one week as shown below.

(i) What observation was made after one week? Explain.
(ii) What property of sulphuric (VI) acid was being investigated in the experiment?
Date posted: March 27, 2019. Answers (1)
- Use the bond energy value given below for the question that follows.
Bond ...(Solved)
Use the bond energy value given below for the question that follows.
Bond Bond energy (kJmol-1)
H – H 432
C = C 610
C – C 346
C – H 413
Determine the enthalpy change for the conversion of butene to butane by hydrogen.
Date posted: March 27, 2019. Answers (1)
- Use the set up below to answer the questions that follow.(Solved)
Use the set up below to answer the questions that follow.
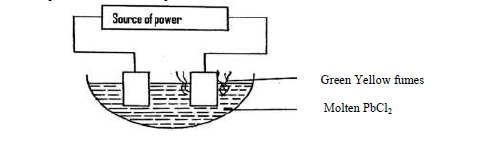
(a) On the diagram, label the cathode.
(b) Write the equation for the reaction on the cathode.
Date posted: March 27, 2019. Answers (1)
- Study the flow chart below and answer the questions that follow.(Solved)
Study the flow chart below and answer the questions that follow.
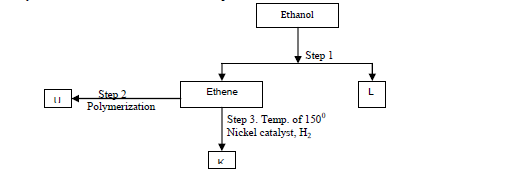
(a) Identify substances: K, U,L
(b) State the conditions for the reaction in step 1 to occur.
(c) Give one disadvantage of continued use of substances such as U.
Date posted: March 27, 2019. Answers (1)
- (a) Define the term oxidation state.
(b) Calculate the oxidation states of chromium and manganese in the following ions.
(i) Chromium in Cr2O72-
(ii) Manganese in...(Solved)
(a) Define the term oxidation state.
(b) Calculate the oxidation states of chromium and manganese in the following ions.
(i) Chromium in Cr2O72-
(ii) Manganese in MnO4-
Date posted: March 27, 2019. Answers (1)