(i)
= 40 (34 – 25) = 40 x 9
= 360 J
(ii)
= 100 x 103 x 4.2 x 103 (34 – 25)
= 3.780J
(iii) = MmCm (100 – 34)
= 0.15cm x 66 = 9.9 cm or
= 360 + 3780
= 4140J
(iv) = 0.15cm x 66 = 4140
= Cm = 4140/ 0.15 x 66 = 418 Jkg-1K-1
Wilfykil answered the question on March 27, 2019 at 12:06
- Figure below shows a toy resting on top of a closed bottle. Use the information on the figure to answer questions below(Solved)
Figure below shows a toy resting on top of a closed bottle. Use the information on the figure to answer questions below

a) Mark on the diagram, point Q, the approximate centre of gravity of the toy.
b) Giving a reason, name the state of equilibrium of the toy.
Date posted: March 27, 2019. Answers (1)
- Water is known to boil at 1000C. A student heated some water and noticed that it boiled at 1010C.
State two possible reasons for this observation(Solved)
Water is known to boil at 1000C. A student heated some water and noticed that it boiled at 1010C.
State two possible reasons for this observation
Date posted: March 27, 2019. Answers (1)
- Figure below shows a brick placed on a plane inclined at an angle ? to the horizontal. The weight, W, of the brick is shown.(Solved)
Figure below shows a brick placed on a plane inclined at an angle θ to the horizontal. The weight, W, of the brick is shown.

a) On the same diagram show with arrows the other two forces acting on the brick and name them.
b) State how each of the two forces named (a) above is affected when the angle θ is reduced.
Date posted: March 27, 2019. Answers (1)
- The masses of equal volumes of a certain liquid and of water were found to be mv and mw respectively. Given that the density of...(Solved)
The masses of equal volumes of a certain liquid and of water were found to be mv and mw respectively. Given that the density of water is 1gcm-3, express the density, p, of the liquid in terms of mv mw (show your work)
Date posted: March 27, 2019. Answers (1)
- The diagram below shows a rectifier circuit for an alternating current (a.c) input.(Solved)
The diagram below shows a rectifier circuit for an alternating current (a.c) input.

Draw the traces of the signal obtained on CRO connected across QS and PR.
Date posted: March 27, 2019. Answers (1)
- You are provided with a diode, a resisitor R, an a.c source of low voltage and connecting wires. In the space provided, sketch the circuit...(Solved)
You are provided with a diode, a resisitor R, an a.c source of low voltage and connecting wires. In the space provided, sketch the circuit diagram for a half-wave rectifier and indicate the terminals where the output voltage v0 may be connected.
Date posted: March 27, 2019. Answers (1)
- Using the components symbols shown in the fig, sketch a series circuit diagram for a forward biased diode.(Solved)
Using the components symbols shown in the fig, sketch a series circuit diagram for a forward biased diode.

Date posted: March 27, 2019. Answers (1)
- Sketch a current-voltage characteristic of a junction diode with a forward bias(Solved)
Sketch a current-voltage characteristic of a junction diode with a forward bias
Date posted: March 27, 2019. Answers (1)
- p- type and n-type semiconductors are made from a pure semiconductor by a process known as “doping”.
i) What is doping?
ii) Explain how the doping produces...(Solved)
p- type and n-type semiconductors are made from a pure semiconductor by a process known as “doping”.
i) What is doping?
ii) Explain how the doping produces an n-type semiconductor.
Date posted: March 27, 2019. Answers (1)
- A radio repairer wishes to use an ammeter to detect a faulty diode. With the aid of a circuit diagram describe how he will go...(Solved)
A radio repairer wishes to use an ammeter to detect a faulty diode. With the aid of a circuit diagram describe how he will go about this task.
Date posted: March 27, 2019. Answers (1)
- Name the quantities, which must be measured so as to determine the half-life of a radioactive sample whose half-life is known to be a few...(Solved)
Name the quantities, which must be measured so as to determine the half-life of a radioactive sample whose half-life is known to be a few hours.
Date posted: March 27, 2019. Answers (1)
- State two factors that determine the extent of the damage to the body cell caused by the radiation from radioactive substances.(Solved)
State two factors that determine the extent of the damage to the body cell caused by the radiation from radioactive substances.
Date posted: March 27, 2019. Answers (1)
- What is meant by the following terms:
Radioactive decay and isotope.(Solved)
What is meant by the following terms:
Radioactive decay and isotope.
Date posted: March 27, 2019. Answers (1)
- A monochromatic beam of radiation is directed on a clean metal surface so as to produce photo-electrons. Give a reason why some of the ejected...(Solved)
A monochromatic beam of radiation is directed on a clean metal surface so as to produce photo-electrons. Give a reason why some of the ejected photo-electrons have more kinetic energy than others.
Date posted: March 27, 2019. Answers (1)
- The diagram shows a photocell in action(Solved)
The diagram shows a photocell in action
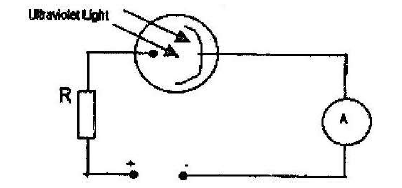
i) The photocell is either evacuated or filled with an inert gas at low pressure. Give one reason for this
ii) What is the function of the resistor R in the circuit?
iii) State one reason for using a particular radiation such as ultraviolet for a given photocell.
iv) Explain how the set-up shown in the diagram may be used as an automatic switching device for a burglar alarm.
Date posted: March 27, 2019. Answers (1)
- Light of frequency 5.5x 1014 HZ is made to strike a surface whose work function is 2.5ev. Show that photoelectric effect will not take place....(Solved)
Light of frequency 5.5x 1014 HZ is made to strike a surface whose work function is 2.5ev. Show that photoelectric effect will not take place. H= 6.6 X 1034Js
Date posted: March 27, 2019. Answers (1)
- State the purpose of cooling fins in the X-ray tube.(Solved)
State the purpose of cooling fins in the X-ray tube.
Date posted: March 27, 2019. Answers (1)
- Explain why X-rays are appropriate in study of the crystalline structure materials.(Solved)
Explain why X-rays are appropriate in study of the crystalline structure materials.
Date posted: March 27, 2019. Answers (1)
- An accelerating potential of 20kv is applied to an X-ray tube.(Solved)
An accelerating potential of 20kv is applied to an X-ray tube.
i) What is the velocity with which the electron strikes the target?
ii) State the energy changes that take place at the target.
Date posted: March 27, 2019. Answers (1)
- For a given source of X-rays, how would the following be controlled.(Solved)
For a given source of X-rays, how would the following be controlled.
i) Intensity
ii) The penetrating power
iii) The exposure to patients
Date posted: March 27, 2019. Answers (1)