(i)S
Because oxygen gas is given out at electrode R thus electrode R is the anode since OH- ions which give oxygen migrate
there.
(ii)4OH-(aq) ……………………>2H2O(l) + O2(g) + 4e-
(iii)A brown solid is deposited on electrode S – this is because the CU2+ ions in solution gain electrons at S to form Copper metal as shown in the following equation.
CU2+(aq) + 2e-……………………>CU(s) brown
sharon kalunda answered the question on March 28, 2019 at 08:51
- The table below gives reduction potentials obtained when the half – cells for each of the metals represented by letters,V,W,X, Y and Z were connected...(Solved)
The table below gives reduction potentials obtained when the half – cells for each of the metals represented by letters,V,W,X, Y and Z were connected to a copper half – cell as the reference electrode.

(a) What is metal X likely to be? Give a reason.
(b) Which of the metals cannot be displaced from the solution of its salt by any other metals in the Table? Give a reason.
Date posted: March 28, 2019. Answers (1)
- Explain why the reaction between 1g of potassium carbonate and 2M HCl is faster than the reaction between 1g sodium carbonate and 2M ethanoic acid.(Solved)
Explain why the reaction between 1g of potassium carbonate and 2M HCl is faster than the reaction between 1g sodium carbonate and 2M ethanoic acid.
Date posted: March 28, 2019. Answers (1)
- Write the equation for the reaction between dilute ethanoic acid and solid sodium carbonate(Solved)
Write the equation for the reaction between dilute ethanoic acid and solid sodium carbonate
Date posted: March 28, 2019. Answers (1)
- Under certain condition ethanoic acid ( C2H4O2) and ethanol ( C2 H5 OH) react to form a sweet smelling compound.
(i) What is the general name...(Solved)
Under certain condition ethanoic acid ( C2H4O2) and ethanol ( C2 H5 OH) react to form a sweet smelling compound.
(i) What is the general name of the compound to which the sweet smelling compound belongs?
(ii) Write the structural formula of the sweet smelling compound
(iii) Give the conditions for the formation of the sweet smelling compound
Date posted: March 28, 2019. Answers (1)
- The structure below represent two cleansing agents(Solved)
The structure below represent two cleansing agents

(a) In the table below,give one advantage and one disadvantage of using each one of them
(b) Which of the two cleaning agents is the better for washing. Explain.

Date posted: March 28, 2019. Answers (1)
- The list below gives the formulae of some organic compounds. Use it to answer the questions that follow.(Solved)
The list below gives the formulae of some organic compounds. Use it to answer the questions that follow.

Select two compounds which:
I. Are not hydrocarbons.
II Belong to the same homologous series
III Identify the compound that is likely to undergo addition polymerization. Give a reason for your answer.
Date posted: March 28, 2019. Answers (1)
- The flow chart below is for the manufacture of sodium carbonate by the Solvay process. Use it to answer the questions that follow.(Solved)
The flow chart below is for the manufacture of sodium carbonate by the Solvay process. Use it to answer the questions that follow.

(i) Name gas M and Q.
(ii) Name solution F and solid X.
(iii) Name the product L formed and give one of its uses.
(iv) Write equations of the reactions in Tower P
Date posted: March 28, 2019. Answers (1)
- A student set up the apparatus shown below to prepare and collect dry carbon (IV) oxide gas.(Solved)
A student set up the apparatus shown below to prepare and collect dry carbon (IV) oxide gas.
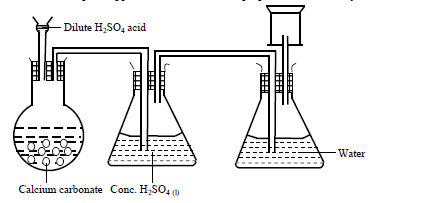
State a correction for three mistakes in the set up above
Date posted: March 28, 2019. Answers (1)
- Study the information in the table below and answer the questions that follow. (The letters are not the actual symbols of the substances)(Solved)
Study the information in the table below and answer the questions that follow. (The letters are not the actual symbols of the substances)

i) Which substance would dissolve in water and could be separated from the solution by fractional distillation? Give a reason.
ii) Which substance is a liquid at room temperature and when mixed with water two layers would be formed?
iii) Which letter represents a substance that is a gas at room temperature and can be collected over water? Explain.
Date posted: March 28, 2019. Answers (1)
- The grid below represents part of the periodic table. Study it and answer the questions that follow. (The letters do not represent the actual symbols...(Solved)
The grid below represents part of the periodic table. Study it and answer the questions that follow. (The letters do not represent the actual symbols of the elements).

i) Select an element which forms a divalent cation.
ii) What type of structure will the chloride of A have?
iii) How do the reactivities of B and E compare? Explain.
iv) Compare the atomic radius of C with that of D. Give a reason for your answer.
v) C and E burn in oxygen to form oxides. Compare the pH values of the solutions of the oxides of C and E.
Date posted: March 28, 2019. Answers (1)
- The half equations involved in a cell are:(Solved)
The half equations involved in a cell are:

(a) Write the overall equation for the electrochemical cell.
(b) Calculate the e.m.f generated by a battery consisting of ten cells.
(c) State one environment advantage of using these cells in spacecrafts
Date posted: March 28, 2019. Answers (1)
- Samples of urine from three participants F, G and H at an international sports meetings were spotted onto chromatography paper alongside two from illegal drugs...(Solved)
Samples of urine from three participants F, G and H at an international sports meetings were spotted onto chromatography paper alongside two from illegal drugs A1 and A2. A chromatogram was run using methanol. The figure below shows the chromatogram.

(a) Identify the athlete who had used an illegal drug.
(b) Which drug is more soluble in methanol?
Date posted: March 28, 2019. Answers (1)
- Zinc reacts with both concentrated and dilute sulphuric (VI) acid. Write equations for the two reactions.(Solved)
Zinc reacts with both concentrated and dilute sulphuric (VI) acid. Write equations for the two reactions.
Date posted: March 28, 2019. Answers (1)
- When a hydrated sample of calcium sulphate CaSO4.xH2O was heated until all the water was lost, the following data was recorded:
Mass of crucible = 30.296...(Solved)
When a hydrated sample of calcium sulphate CaSO4.xH2O was heated until all the water was lost, the following data was recorded:
Mass of crucible = 30.296 g
Mass of crucible + hydrated salt = 33.111 g
Mass of crucible + anhydrous salt = 32.781 g
Determine the empirical formula of the hydrated salt
(CA = 40, S = 32, O = 16 H = 1)
Date posted: March 28, 2019. Answers (1)
- Molecular substances have low melting points. Give one reason why they have low melting points.(Solved)
Molecular substances have low melting points. Give one reason why they have low melting points.
Date posted: March 28, 2019. Answers (1)
- State the type of bond that exists between the NH4+ ion and H+ ion.(Solved)
State the type of bond that exists between the NH4+ ion and H+ ion.
Date posted: March 28, 2019. Answers (1)
- Using a dot (.) and cross (x) show how NH4+ ion is formed from NH3 molecule and H+ ion.(Solved)
Using a dot (.) and cross (x) show how NH4+ ion is formed from NH3 molecule and H+ ion.
Date posted: March 28, 2019. Answers (1)
- The set up of diagram shown below is used to prepare dry nitrogen gas from air. Study it and answer the questions that follow.(Solved)
The set up of diagram shown below is used to prepare dry nitrogen gas from air. Study it and answer the questions that follow.

(a) What is the purpose of using:
(i) A burning candle.
(ii) Sodium hydroxide solution.
(b) Name:
(i) One impurity present in nitrogen gas prepared.
(ii) A suitable drying agent used.
Date posted: March 28, 2019. Answers (1)
- The set-up below was used to prepare dry chlorine gas. Study and answer the questions that follow(Solved)
The set-up below was used to prepare dry chlorine gas. Study and answer the questions that follow

(a) Name reagents M and substance L.
(b) A warm red phosphorus was lowered into the gas jar of chlorine using a deflagrating spoon:
(i) State any one observation made in this experiment.
(ii) Identify the substance formed in the above reaction.
(c) Both substances in (ii) above undergo hydrolysis when exposed to air. Write an equation to show how anyone of them undergoes hydrolysis.
Date posted: March 28, 2019. Answers (1)
- To which homologous series does the compound below belong?(Solved)
To which homologous series does the compound below belong?

Date posted: March 28, 2019. Answers (1)