(a) Yellow solid is formed.
SO2 gas is reduced by H2S to sulphur.
(b)-Jars should be moist.
-The jar with the denser gas should be placed on top of the jar with the light gas.
(c) 2H2S(aq) + SO(g)------>2H2O(l) + 3S(s)
(d) Oxidizing agent
sharon kalunda answered the question on March 28, 2019 at 10:51
- The table below shows some solutions and their PH values.(Solved)
The table below shows some solutions and their PH values.
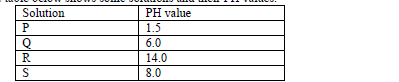
Which of the above solution.
(a) Is strongly basic.
(b) Reacts with sodium carbonate more vigorously.
(c) Is ammonia solution.
Date posted: March 28, 2019. Answers (1)
- The diagram below represents a set-up used to prepare oxygen gas.(Solved)
The diagram below represents a set-up used to prepare oxygen gas.

(a) Name substance Q.
(b) Complete the set-up to show how oxygen gas is collected.
(c) Write the equation for the reaction that occur.
Date posted: March 28, 2019. Answers (1)
- Describe briefly how potassium sulphate can be prepared using 50cm³ of 1M potassium hydroxide.(Solved)
Describe briefly how potassium sulphate can be prepared using 50cm³ of 1M potassium hydroxide.
Date posted: March 28, 2019. Answers (1)
- What is meant by the term basicity of an acid.(Solved)
What is meant by the term basicity of an acid.
Date posted: March 28, 2019. Answers (1)
- When an electric current of 0.5A was passed through a molten chloride of J for 32 minutes and 10 seconds, a mass of 0.44g of...(Solved)
When an electric current of 0.5A was passed through a molten chloride of J for 32 minutes and 10 seconds, a mass of 0.44g of J was deposited at the cathode. (IF = 96500C).
(a) Calculate the quantity of electricity used.
(b) Determine the value of x if the ion of metal J is represented as Jx+.
(R.A.M of J = 44).
Date posted: March 28, 2019. Answers (1)
- Study the information below and answer the questions that follow.(Solved)
Study the information below and answer the questions that follow.
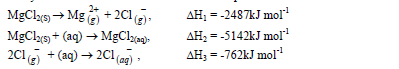
(a) Name the enthalpies H1 and H2.
(b) Determine the enthalpy for the reaction:

Date posted: March 28, 2019. Answers (1)
- Using electrons in the outermost energy level, draw the dot (.) and cross (X) diagrams to represent bonding in.
(Solved)
Using electrons in the outermost energy level, draw the dot (.) and cross (X) diagrams to represent bonding in.
a)(i) C2H6 (C = 6, H = 1)
(ii) NH4Cl (N = 7, H = l, Cl = 17)
b)The formula of a complex ion is  name the type of bond that is likely to exist between copper and
name the type of bond that is likely to exist between copper and
ammonia in the complex.
Date posted: March 28, 2019. Answers (1)
- The mass of a solution A is 120g. This solution has 8g of salt A dissolved in it. The solubility of this salt is 25g/100g...(Solved)
The mass of a solution A is 120g. This solution has 8g of salt A dissolved in it. The solubility of this salt is 25g/100g of water at 300C. 55g of salt A are added to the solution at 300C. How much of salt A will remain undissolved.
Date posted: March 28, 2019. Answers (1)
- A certain carbonate,JCO3, reacts with dilute hydrochloric acid according to the equation below.(Solved)
A certain carbonate, JCO3, reacts with dilute hydrochloric acid according to the equation below.
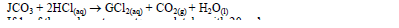
If 1gof the carbonate reacts completely with 20cm³ of 1M hydrochloric acid, calculate the relative atomic mass of J. (C = 12, O = 16).
Date posted: March 28, 2019. Answers (1)
- The thermodynamic equation for the formation of ammonia in the Haber process is:(Solved)
The thermodynamic equation for the formation of ammonia in the Haber process is:

State and explain one way in which the yield of ammonia can be increased.
Date posted: March 28, 2019. Answers (1)
- A metal oxide has a formula M2 O3.
(a) Write an equation to show how M form an ion.
(b) Write the formula of the chloride...(Solved)
A metal oxide has a formula M2 O3.
(a) Write an equation to show how M form an ion.
(b) Write the formula of the chloride of M.
Date posted: March 28, 2019. Answers (1)
- If after 112 days 1/16 of polonium remained, calculate the half-life of polonium.(Solved)
If after 112 days 1/16 of polonium remained, calculate the half-life of polonium.
Date posted: March 28, 2019. Answers (1)
- Radioactive polonium – 216 decay as shown below.(Solved)
Radioactive polonium – 216 decay as shown below.

Find the value of M and n.
Date posted: March 28, 2019. Answers (1)
- The flow chart below gives some reactions starting with ethane. Study it and answer the questions that follow.(Solved)
The flow chart below gives some reactions starting with ethane. Study it and answer the questions that follow.
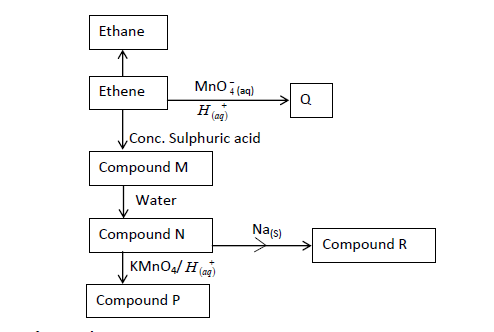
(a) Draw the structure of compounds:
P:
Q:
(b) Write the name of Compound R.
Date posted: March 28, 2019. Answers (1)
- An approximately X molar solution of potassium managanate (VII) solution was standardized against precisely 0.1M iron (II) ammonium sulphate [(NH4)3 Fe (SO4)2. 6H2O] solution. 25.0cm³...(Solved)
An approximately X molar solution of potassium managanate (VII) solution was standardized against precisely 0.1M iron (II) ammonium sulphate [(NH4)3 Fe (SO4)2. 6H2O] solution. 25.0cm³ of the solution of the iron (II) salt were oxidized by 24.15cm³ of the manganate (VII) solution. The equation of the reaction is:
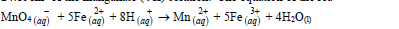
What is the molarity of the potassium manganate (III) solution?
Date posted: March 28, 2019. Answers (1)
- An ion of element X is represented as:(Solved)
An ion of element X is represented as:

(i) Write electronic configuration of ion of X.
(ii) To which group does element X belong?
Date posted: March 28, 2019. Answers (1)
- The apparatus below was used to separate a mixture of liquid A and B.(Solved)
The apparatus below was used to separate a mixture of liquid A and B.
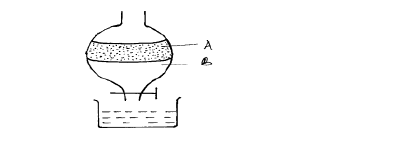
(i) State two properties of the liquids that make it possible to separate them are using such apparatus.
(ii) Give the name of the above apparatus.
Date posted: March 28, 2019. Answers (1)
- Two samples of equal volumes of water were put in 250cm³ beaker and heated for 10 minutes. Sample 1 registered a higher temperature than sample...(Solved)
Two samples of equal volumes of water were put in 250cm³ beaker and heated for 10 minutes. Sample 1 registered a higher temperature than sample 2.
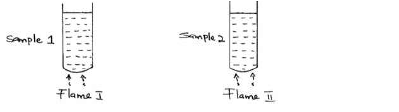
State the conditions under which flame I is produced in Bunsen burner.
Date posted: March 28, 2019. Answers (1)
- A patient was given tablets with prescription 2 x 3 on the envelope. Clearly outline how the patient should take the tablets.(Solved)
A patient was given tablets with prescription 2 x 3 on the envelope. Clearly outline how the patient should take the tablets.
Date posted: March 28, 2019. Answers (1)
- Study the following reaction scheme for the extraction of zinc metal and then answer the questions that follow.(Solved)
Study the following reaction scheme for the extraction of zinc metal and then answer the questions that follow.
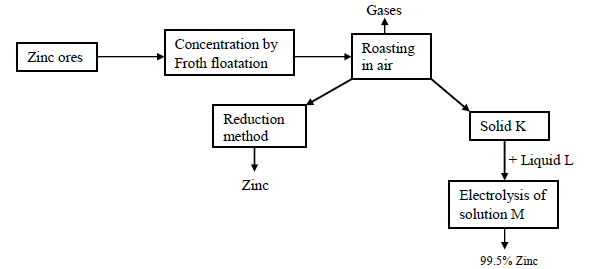
(a) Name two chief ores from which zinc can be extracted
(b) (i) Name the reducing agents used in the reduction chamber.
(ii) Write the equations for the reduction process to obtain zinc
(c) Identify the following:
Solid K
Liquid L
Date posted: March 28, 2019. Answers (1)