Place the mixture in a beaker with hot water and stir. Filter the mixture while hot to obtain solid B as a residue. Let the filtrate containing A and C cool. Solid C will be deposited since it is
insoluble in cold water. Filter to obtain solid C as a residue. Evaporate the filtrate to dryness to
obtain solid A.
Kavungya answered the question on March 28, 2019 at 11:53
- Study the diagram below hence answer the questions that follow.(Solved)
Study the diagram below hence answer the questions that follow.
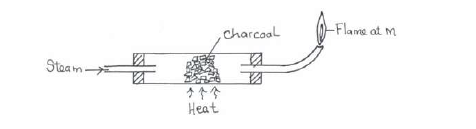
(i) Explain why it is necessary to have the flame at M.
(ii) Write down the equation for the reaction inside the apparatus.
Date posted: March 28, 2019. Answers (1)
- Calculate the volume of carbon (IV) oxide that would be produced if 15g of calcium carbonate reacted with 100cm³ of 2.0M hydrochloric acid (C =...(Solved)
Calculate the volume of carbon (IV) oxide that would be produced if 15g of calcium carbonate reacted with 100cm³ of 2.0M hydrochloric acid (C = 12.0, O = 16.0, Ca = 40.0) molar gas volume = 24000cm³
Date posted: March 28, 2019. Answers (1)
- When lead (II) Carbonate is reacted with dilute sulphuric (VI) acid, the reaction takes place for a short time and then stops. Explain.(Solved)
When lead (II) Carbonate is reacted with dilute sulphuric (VI) acid, the reaction takes place for a short time and then stops. Explain.
Date posted: March 28, 2019. Answers (1)
- Name the particles responsible for the electrical conductivity of:
(a) Graphite
(b) Magnesium Sulphate solution(Solved)
Name the particles responsible for the electrical conductivity of:
(a) Graphite
(b) Magnesium Sulphate solution
Date posted: March 28, 2019. Answers (1)
- The diagram below illustrates how lithium would react with steam. Study it then answer the questions that follow.(Solved)
The diagram below illustrates how lithium would react with steam. Study it then answer the questions that follow.

(a) Write the equation for the reaction that takes place.
(b) Explain why this experiment cannot be carried out with potassium in the same way as shown.
Date posted: March 28, 2019. Answers (1)
- The melting point of phosphorous (III) chloride is -910C while that of magnesium chloride is +7150C. In terms of structure and bonding explain the difference.(Solved)
The melting point of phosphorous (III) chloride is -910C while that of magnesium chloride is +7150C. In terms of structure and bonding explain the difference.
Date posted: March 28, 2019. Answers (1)
- The atomic numbers of nitrogen, oxygen and sodium are 7, 8 and 11 respectively.
(a) Write the electron arrangements of their ions, N3-, O2- and Na+....(Solved)
The atomic numbers of nitrogen, oxygen and sodium are 7, 8 and 11 respectively.
(a) Write the electron arrangements of their ions, N3-, O2- and Na+.
(b) Arrange the 3 ions in increasing order of size. Give a reason for your answer.
Date posted: March 28, 2019. Answers (1)
- The grid below is part of the periodic table. Study it and answer the questions that follow. The letters are not actual symbols of elements.(Solved)
The grid below is part of the periodic table. Study it and answer the questions that follow. The letters are not actual symbols of elements.
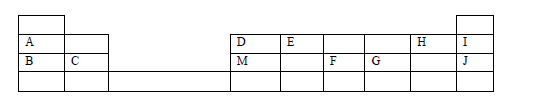
(a) What is the name given to the chemical family of element C?
(b) Would element B react with J? Explain.
(c) Compare the melting points of B and M.
Date posted: March 28, 2019. Answers (1)
- It is advisable to leave your flame in the luminous state when not in use. Give a reason why.(Solved)
It is advisable to leave your flame in the luminous state when not in use. Give a reason why.
Date posted: March 28, 2019. Answers (1)
- The diagram below shows a non-luminous flame. Use it to answer the questions that follow:(Solved)
The diagram below shows a non-luminous flame. Use it to answer the questions that follow:
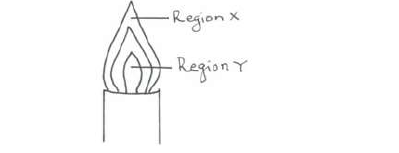
Two wooden splints were placed across regions X and Y respectively. Draw labelled diagrams to show the effects observed on the wooden splint placed across each region.
(i) Region X.
(ii) Region Y.
Date posted: March 28, 2019. Answers (1)
- The table below shows PH values of solutions A, B, C and D.(Solved)
The table below shows PH values of solutions A, B, C and D.

(a) Identify a solution which is
(i) Strongly acidic. _________________________________________
(ii) Strongly basic _________________________________________
(b) Which to solutions would react with lead (II) oxide? Explain.
Date posted: March 28, 2019. Answers (1)
- Write the equation for decomposition of:
(a) Sodium nitrate.
(b) Copper (II) nitrate.(Solved)
Write the equation for decomposition of:
(a) Sodium nitrate.
(b) Copper (II) nitrate.
Date posted: March 28, 2019. Answers (1)
- An iron spoon was to be electroplated with silver. Sketch the set-up that could be used.(Solved)
An iron spoon was to be electroplated with silver. Sketch the set-up that could be used.
Date posted: March 28, 2019. Answers (1)
- 280cm³ of nitrogen gas diffuse through a porous plug in 70 seconds. How long will it take 400cm³ of carbon (IV) oxide gas to diffuse...(Solved)
280cm³ of nitrogen gas diffuse through a porous plug in 70 seconds. How long will it take 400cm³ of carbon (IV) oxide gas to diffuse through the same porous plug. (C = 12, O = 16, N = 7).
Date posted: March 28, 2019. Answers (1)
- Two reagents that can be used to prepare chlorine gas are potassium manganate (VII) and hydrochloric acid.
(a) Write an equation for the reaction.
(b) Give...(Solved)
Two reagents that can be used to prepare chlorine gas are potassium manganate (VII) and hydrochloric acid.
(a) Write an equation for the reaction.
(b) Give the formula of another reagent that can be used instead of potassium manganate (VII).
(c) Using an equation illustrate how chlorine bleach coloured substances.
Date posted: March 28, 2019. Answers (1)
- In an experiment, a jar containing sulphur (IV) oxide was inverted over another jar containing hydrogen sulphide gas.
(a) State and explain the observation that was...(Solved)
In an experiment, a jar containing sulphur (IV) oxide was inverted over another jar containing hydrogen sulphide gas.
(a) State and explain the observation that was made.
(b) State two conditions necessary for the reaction to take place.
(c) Write an equation for the reaction
(d) What is the role of sulphur (IV) oxide in the reaction
Date posted: March 28, 2019. Answers (1)
- The table below shows some solutions and their PH values.(Solved)
The table below shows some solutions and their PH values.
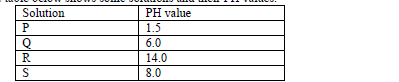
Which of the above solution.
(a) Is strongly basic.
(b) Reacts with sodium carbonate more vigorously.
(c) Is ammonia solution.
Date posted: March 28, 2019. Answers (1)
- The diagram below represents a set-up used to prepare oxygen gas.(Solved)
The diagram below represents a set-up used to prepare oxygen gas.

(a) Name substance Q.
(b) Complete the set-up to show how oxygen gas is collected.
(c) Write the equation for the reaction that occur.
Date posted: March 28, 2019. Answers (1)
- Describe briefly how potassium sulphate can be prepared using 50cm³ of 1M potassium hydroxide.(Solved)
Describe briefly how potassium sulphate can be prepared using 50cm³ of 1M potassium hydroxide.
Date posted: March 28, 2019. Answers (1)
- What is meant by the term basicity of an acid.(Solved)
What is meant by the term basicity of an acid.
Date posted: March 28, 2019. Answers (1)