-
A Student in form four placed a thermometer in molten naphthalene at 850C and recorded the temperature and time until the naphthalene solidified. From the...
(Solved)
A Student in form four placed a thermometer in molten naphthalene at 850C and recorded the temperature and time until the naphthalene solidified. From the values obtained, the figure below was drawn.
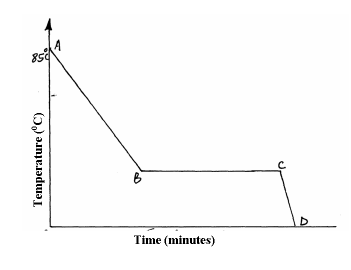
(a) What name is given to such a figure?
(b) Which part of the figure represents the change of state of naphthalene?
(c)In terms of kinetic theory. Explain what happens to molecules along AB.
Date posted:
March 28, 2019
.
Answers (1)
-
The table below shows information about three solid substances A, B and C. Study it and answer the question that follow.
Describe how you will separate...
(Solved)
The table below shows information about three solid substances A, B and C. Study it and answer the question that follow.
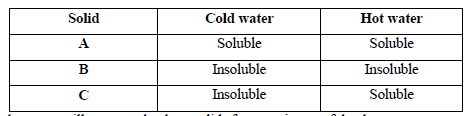
Describe how you will separate the three solids from a mixture of the three.
Date posted:
March 28, 2019
.
Answers (1)
-
Calculate the volume of carbon (IV) oxide that would be produced if 15g of calcium carbonate reacted with 100cm³ of 2.0M hydrochloric acid (C =...
(Solved)
Calculate the volume of carbon (IV) oxide that would be produced if 15g of calcium carbonate reacted with 100cm³ of 2.0M hydrochloric acid (C = 12.0, O = 16.0, Ca = 40.0) molar gas volume = 24000cm³
Date posted:
March 28, 2019
.
Answers (1)
-
When lead (II) Carbonate is reacted with dilute sulphuric (VI) acid, the reaction takes place for a short time and then stops. Explain.
(Solved)
When lead (II) Carbonate is reacted with dilute sulphuric (VI) acid, the reaction takes place for a short time and then stops. Explain.
Date posted:
March 28, 2019
.
Answers (1)
-
Name the particles responsible for the electrical conductivity of:
(a) Graphite
(b) Magnesium Sulphate solution
(Solved)
Name the particles responsible for the electrical conductivity of:
(a) Graphite
(b) Magnesium Sulphate solution
Date posted:
March 28, 2019
.
Answers (1)
-
It is advisable to leave your flame in the luminous state when not in use. Give a reason why.
(Solved)
It is advisable to leave your flame in the luminous state when not in use. Give a reason why.
Date posted:
March 28, 2019
.
Answers (1)
-
The diagram below shows a non-luminous flame. Use it to answer the questions that follow:
(Solved)
The diagram below shows a non-luminous flame. Use it to answer the questions that follow:
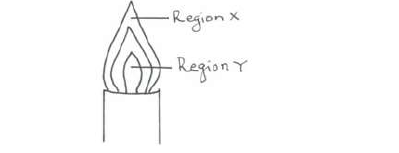
Two wooden splints were placed across regions X and Y respectively. Draw labelled diagrams to show the effects observed on the wooden splint placed across each region.
(i) Region X.
(ii) Region Y.
Date posted:
March 28, 2019
.
Answers (1)
-
280cm³ of nitrogen gas diffuse through a porous plug in 70 seconds. How long will it take 400cm³ of carbon (IV) oxide gas to diffuse...
(Solved)
280cm³ of nitrogen gas diffuse through a porous plug in 70 seconds. How long will it take 400cm³ of carbon (IV) oxide gas to diffuse through the same porous plug. (C = 12, O = 16, N = 7).
Date posted:
March 28, 2019
.
Answers (1)
-
In an experiment, a jar containing sulphur (IV) oxide was inverted over another jar containing hydrogen sulphide gas.
(a) State and explain the observation that was...
(Solved)
In an experiment, a jar containing sulphur (IV) oxide was inverted over another jar containing hydrogen sulphide gas.
(a) State and explain the observation that was made.
(b) State two conditions necessary for the reaction to take place.
(c) Write an equation for the reaction
(d) What is the role of sulphur (IV) oxide in the reaction
Date posted:
March 28, 2019
.
Answers (1)
-
The diagram below represents a set-up used to prepare oxygen gas.
(Solved)
The diagram below represents a set-up used to prepare oxygen gas.

(a) Name substance Q.
(b) Complete the set-up to show how oxygen gas is collected.
(c) Write the equation for the reaction that occur.
Date posted:
March 28, 2019
.
Answers (1)
-
Describe briefly how potassium sulphate can be prepared using 50cm³ of 1M potassium hydroxide.
(Solved)
Describe briefly how potassium sulphate can be prepared using 50cm³ of 1M potassium hydroxide.
Date posted:
March 28, 2019
.
Answers (1)
-
Radioactive polonium – 216 decay as shown below.
(Solved)
Radioactive polonium – 216 decay as shown below.

Find the value of M and n.
Date posted:
March 28, 2019
.
Answers (1)
-
The flow chart below gives some reactions starting with ethane. Study it and answer the questions that follow.
(Solved)
The flow chart below gives some reactions starting with ethane. Study it and answer the questions that follow.
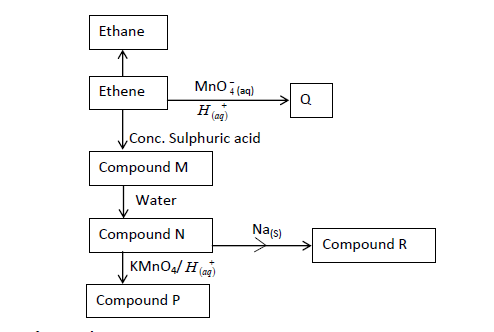
(a) Draw the structure of compounds:
P:
Q:
(b) Write the name of Compound R.
Date posted:
March 28, 2019
.
Answers (1)
-
The apparatus below was used to separate a mixture of liquid A and B.
(Solved)
The apparatus below was used to separate a mixture of liquid A and B.
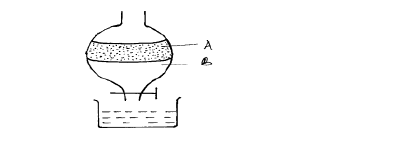
(i) State two properties of the liquids that make it possible to separate them are using such apparatus.
(ii) Give the name of the above apparatus.
Date posted:
March 28, 2019
.
Answers (1)
-
Two samples of equal volumes of water were put in 250cm³ beaker and heated for 10 minutes. Sample 1 registered a higher temperature than sample...
(Solved)
Two samples of equal volumes of water were put in 250cm³ beaker and heated for 10 minutes. Sample 1 registered a higher temperature than sample 2.
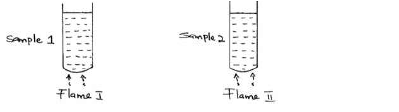
State the conditions under which flame I is produced in Bunsen burner.
Date posted:
March 28, 2019
.
Answers (1)
-
A patient was given tablets with prescription 2 x 3 on the envelope. Clearly outline how the patient should take the tablets.
(Solved)
A patient was given tablets with prescription 2 x 3 on the envelope. Clearly outline how the patient should take the tablets.
Date posted:
March 28, 2019
.
Answers (1)
-
Study the following reaction scheme for the extraction of zinc metal and then answer the questions that follow.
(Solved)
Study the following reaction scheme for the extraction of zinc metal and then answer the questions that follow.
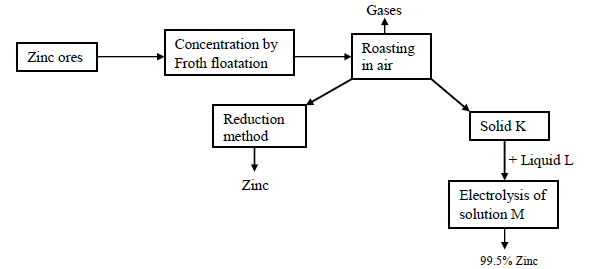
(a) Name two chief ores from which zinc can be extracted
(b) (i) Name the reducing agents used in the reduction chamber.
(ii) Write the equations for the reduction process to obtain zinc
(c) Identify the following:
Solid K
Liquid L
Date posted:
March 28, 2019
.
Answers (1)
-
The table below gives reduction potentials obtained when the half – cells for each of the metals represented by letters,V,W,X, Y and Z were connected...
(Solved)
The table below gives reduction potentials obtained when the half – cells for each of the metals represented by letters,V,W,X, Y and Z were connected to a copper half – cell as the reference electrode.

(a) What is metal X likely to be? Give a reason.
(b) Which of the metals cannot be displaced from the solution of its salt by any other metals in the Table? Give a reason.
Date posted:
March 28, 2019
.
Answers (1)
-
Explain why the reaction between 1g of potassium carbonate and 2M HCl is faster than the reaction between 1g sodium carbonate and 2M ethanoic acid.
(Solved)
Explain why the reaction between 1g of potassium carbonate and 2M HCl is faster than the reaction between 1g sodium carbonate and 2M ethanoic acid.
Date posted:
March 28, 2019
.
Answers (1)
-
The flow chart below is for the manufacture of sodium carbonate by the Solvay process. Use it to answer the questions that follow.
(Solved)
The flow chart below is for the manufacture of sodium carbonate by the Solvay process. Use it to answer the questions that follow.

(i) Name gas M and Q.
(ii) Name solution F and solid X.
(iii) Name the product L formed and give one of its uses.
(iv) Write equations of the reactions in Tower P
Date posted:
March 28, 2019
.
Answers (1)