KCl contains mobile ions in both state while HCl does not Contain mobile ions in its gaseous state but dissociate in 3
Aqueous state to form mobile ions
Kavungya answered the question on March 28, 2019 at 12:30
- A certain substance has a boiling point of 16800C. It does not conduct electricity when in solid form but conducts when molten. What is the...(Solved)
A certain substance has a boiling point of 16800C. It does not conduct electricity when in solid form but conducts when molten. What is the most likely structure of the substance? Explain.
Date posted: March 28, 2019. Answers (1)
- Study the diagrams below.(Solved)
Study the diagrams below.
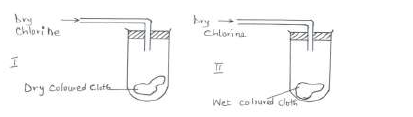
(a) State the observations made at I and II.
(b) Write the equation to show the reactions at II if dry sulphur (IV) oxide was used in place of dry chlorine.
Date posted: March 28, 2019. Answers (1)
- The table below shows elements W, X, Y and Z and their atomic numbers. The letters are not the actual symbols of the elements. Use...(Solved)
The table below shows elements W, X, Y and Z and their atomic numbers. The letters are not the actual symbols of the elements. Use the letters to answer the questions that follow.
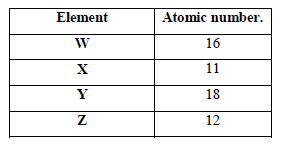
(a) Select an element which forms
(i) Anions.
(ii) An insoluble carbonate
(b) Which element has the largest atomic radius
Date posted: March 28, 2019. Answers (1)
- Briefly explain the following
(a) Atomic radii of alkaline earth metals are smaller than those of the corresponding alkali metals in the same period.
(b) Melting point...(Solved)
Briefly explain the following
(a) Atomic radii of alkaline earth metals are smaller than those of the corresponding alkali metals in the same period.
(b) Melting point of halogens increase down the group.
(c) Helium is a better gas for use in weather research balloons than hydrogen.
Date posted: March 28, 2019. Answers (1)
- An element E has relative atomic mass of 69.39. Given that the element has two isotopes of atomic masses 62.35 and 70.45, calculate the relative...(Solved)
An element E has relative atomic mass of 69.39. Given that the element has two isotopes of atomic masses 62.35 and 70.45, calculate the relative abundance of each of the isotopes.
Date posted: March 28, 2019. Answers (1)
- Study the diagram below and answer the questions that follow.
(a) Identify gas Z.
(b) Write the chemical equation for the reaction which produces gas Z
(c)...(Solved)
Study the diagram below and answer the questions that follow.
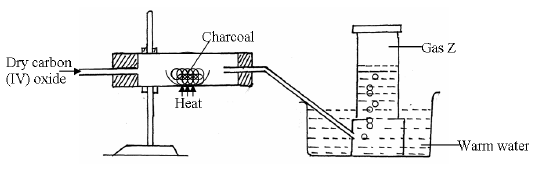
(a) Identify gas Z.
(b) Write the chemical equation for the reaction which produces gas Z
(c) State why the above experiment should be carried out in a fume chamber.
Date posted: March 28, 2019. Answers (1)
- Study the scheme below hence answer the questions that follow.(Solved)
Study the scheme below hence answer the questions that follow.
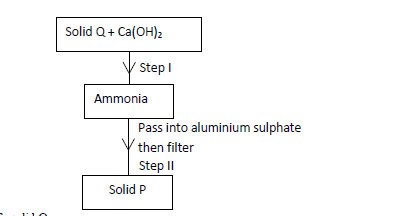
(a) Identify solid Q.
(b) Write an ionic equation for the reaction in Step II.
(c) State the condition necessary for Step I.
Date posted: March 28, 2019. Answers (1)
- Name the method of separation that can most suitably be used to separate the following mixtures.
(a) Gasoline from petroleum
(b) Benzoic acid and potassium carbonate
(c) Oil...(Solved)
Name the method of separation that can most suitably be used to separate the following mixtures.
(a) Gasoline from petroleum
(b) Benzoic acid and potassium carbonate
(c) Oil from cashew nuts
Date posted: March 28, 2019. Answers (1)
- Study the set-up below and answer the questions that follow.
After sometimes, the cotton wools X, Y and Z changed color in turn.
(a) What were the...(Solved)
Study the set-up below and answer the questions that follow.
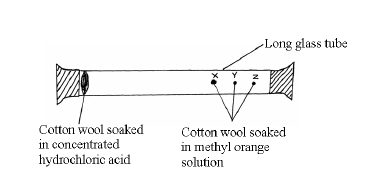
After sometimes, the cotton wools X, Y and Z changed color in turn.
(a) What were the colour changes?
(b) Which cotton wool changed colour first?
(c) Explain why the cotton wools did not change colour at the same time.
Date posted: March 28, 2019. Answers (1)
- A current of 2.5A was passed through a cell containing N2+ ions for 25 minutes. The mass of the cathode increased by 0.36g.Determine the R.A.M...(Solved)
A current of 2.5A was passed through a cell containing N2+ ions for 25 minutes. The mass of the cathode increased by 0.36g.Determine the R.A.M of N. (F = 9.65 x 104Cmolˉ¹).
Date posted: March 28, 2019. Answers (1)
- The diagram below represents a set-up of apparatus used to investigate the effect of electric current on lead (II) bromide.(Solved)
The diagram below represents a set-up of apparatus used to investigate the effect of electric current on lead (II) bromide.
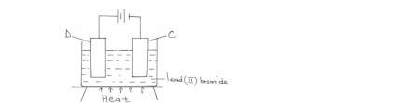
Describe what is observed at electrode C.
Date posted: March 28, 2019. Answers (1)
- 25.0cm3 of 0.12M potassium hydroxide solution required 30.0cm3 of a solution of a dibasic acid (H2Y) for complete neutralization. The acid contained 3.15g per 500cm3...(Solved)
25.0cm3 of 0.12M potassium hydroxide solution required 30.0cm3 of a solution of a dibasic acid (H2Y) for complete neutralization. The acid contained 3.15g per 500cm3 solution.
Calculate:
(a) The molarity of the acid solution
(b) The relative formula mass of the acid.
Date posted: March 28, 2019. Answers (1)
- The table below contains information regarding a species of helium.(Solved)
The table below contains information regarding a species of helium.

Complete the table by indicating the numbers of electrons and neutrons.
Date posted: March 28, 2019. Answers (1)
- A Student in form four placed a thermometer in molten naphthalene at 850C and recorded the temperature and time until the naphthalene solidified. From the...(Solved)
A Student in form four placed a thermometer in molten naphthalene at 850C and recorded the temperature and time until the naphthalene solidified. From the values obtained, the figure below was drawn.
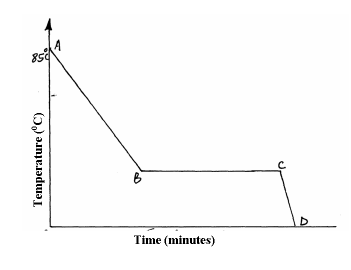
(a) What name is given to such a figure?
(b) Which part of the figure represents the change of state of naphthalene?
(c)In terms of kinetic theory. Explain what happens to molecules along AB.
Date posted: March 28, 2019. Answers (1)
- The table below shows information about three solid substances A, B and C. Study it and answer the question that follow.
Describe how you will separate...(Solved)
The table below shows information about three solid substances A, B and C. Study it and answer the question that follow.
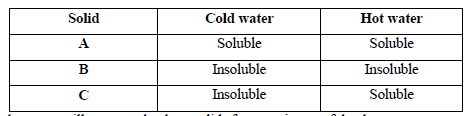
Describe how you will separate the three solids from a mixture of the three.
Date posted: March 28, 2019. Answers (1)
- Study the diagram below hence answer the questions that follow.(Solved)
Study the diagram below hence answer the questions that follow.
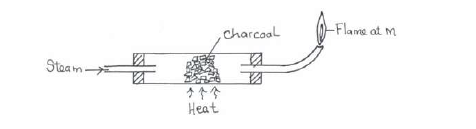
(i) Explain why it is necessary to have the flame at M.
(ii) Write down the equation for the reaction inside the apparatus.
Date posted: March 28, 2019. Answers (1)
- Calculate the volume of carbon (IV) oxide that would be produced if 15g of calcium carbonate reacted with 100cm³ of 2.0M hydrochloric acid (C =...(Solved)
Calculate the volume of carbon (IV) oxide that would be produced if 15g of calcium carbonate reacted with 100cm³ of 2.0M hydrochloric acid (C = 12.0, O = 16.0, Ca = 40.0) molar gas volume = 24000cm³
Date posted: March 28, 2019. Answers (1)
- When lead (II) Carbonate is reacted with dilute sulphuric (VI) acid, the reaction takes place for a short time and then stops. Explain.(Solved)
When lead (II) Carbonate is reacted with dilute sulphuric (VI) acid, the reaction takes place for a short time and then stops. Explain.
Date posted: March 28, 2019. Answers (1)
- Name the particles responsible for the electrical conductivity of:
(a) Graphite
(b) Magnesium Sulphate solution(Solved)
Name the particles responsible for the electrical conductivity of:
(a) Graphite
(b) Magnesium Sulphate solution
Date posted: March 28, 2019. Answers (1)
- The diagram below illustrates how lithium would react with steam. Study it then answer the questions that follow.(Solved)
The diagram below illustrates how lithium would react with steam. Study it then answer the questions that follow.

(a) Write the equation for the reaction that takes place.
(b) Explain why this experiment cannot be carried out with potassium in the same way as shown.
Date posted: March 28, 2019. Answers (1)