I Hydrogen
II Oxygen
sharon kalunda answered the question on March 28, 2019 at 12:56
- When bromine gas reacts with aqueous sodium hydroxide an equilibrium is established as shown below.(Solved)
When bromine gas reacts with aqueous sodium hydroxide an equilibrium is established as shown below.
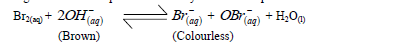
What observation would be made if a few drops of dilute sulphuric (VI) were added to the equilibrium mixture? Explain.
Date posted: March 28, 2019. Answers (1)
- Butane burns in air according to the equation shown below.
2C4H10(g) + 13O2(g) ----> 8CO2(g) + 10H2O(l)
What volume of butane must be burnt in oxygen to...(Solved)
Butane burns in air according to the equation shown below.
2C4H10(g) + 13O2(g) ----> 8CO2(g) + 10H2O(l)
What volume of butane must be burnt in oxygen to give 11g of CO2 at r.t.p?
(Molar gas volume at r.t.p = 24.0L; C = 120; O = 16.0; H = 1.0 )
Date posted: March 28, 2019. Answers (1)
- Study the cycle below hence answer the questions that follow.(Solved)
Study the cycle below hence answer the questions that follow.
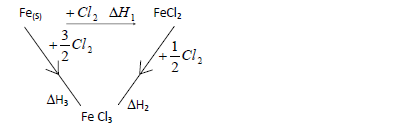
(a) What is (change)H3?
(b) Show the relationship connecting (change)H1,(change)H2 and (change)H3
Date posted: March 28, 2019. Answers (1)
- A gas of known mass occupies 200cm3 at 250C and 101325 Pa pressure. What volume would it occupy
at -230C and 1000 Pa pressure?(Solved)
A gas of known mass occupies 200cm3 at 250C and 101325 Pa pressure. What volume would it occupy
at -230C and 1000 Pa pressure?
Date posted: March 28, 2019. Answers (1)
- In an experiment soap solution was used against 3 separate samples of water. Each sample was later boiled and soap added. Each water sample was...(Solved)
In an experiment soap solution was used against 3 separate samples of water. Each sample was later boiled and soap added.Each water sample was 1000cm³. The results are tabulated below.
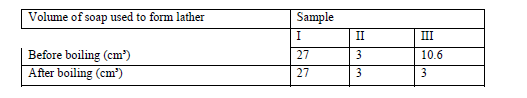
(a) Which sample was likely to be soft water? Explain.
(b) State the cause of change in volume of soap used to form lather in sample III.
Date posted: March 28, 2019. Answers (1)
- The electronic configuration of element X, Y and Z are given below.
X Y ...(Solved)
The electronic configuration of element X, Y and Z are given below.
X Y Z
2.8 2.8.7 2.8.18.7
Which is the most reactive element? Explain.
Date posted: March 28, 2019. Answers (1)
- The scheme below shows some reaction of salt. Study it and answer questions that follow.(Solved)
The scheme below shows some reaction of salt. Study it and answer questions that follow.
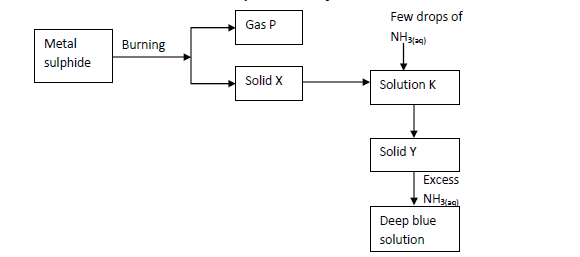
(i) Write an equation for the reaction to show formation of gas P and solid X.
(ii) Give the name and formula of the complex ion responsible for the deep blue colour in the solution.
Date posted: March 28, 2019. Answers (1)
- Excess carbon II Oxide was passed over heated sample of an oxide of iron. The following
results were obtained. Determine the empirical formular of the iron...(Solved)
Excess carbon II Oxide was passed over heated sample of an oxide of iron. The following
results were obtained. Determine the empirical formular of the iron oxide.
Mass of empty dish = 10.98g
Mass of empty dish + oxide of iron = 13.30g
Mass of dish + residue = 12.66g
(Fe = 56; C = 12; O = 16 )
Date posted: March 28, 2019. Answers (1)
- Explain why potassium chloride conducts electricity in both the molten state and in aqueous solution whereas hydrogen chloride conducts electricity only in aqueous solution and...(Solved)
Explain why potassium chloride conducts electricity in both the molten state and in aqueous solution whereas hydrogen chloride conducts electricity only in aqueous solution and not in gaseous state.
Date posted: March 28, 2019. Answers (1)
- A certain substance has a boiling point of 16800C. It does not conduct electricity when in solid form but conducts when molten. What is the...(Solved)
A certain substance has a boiling point of 16800C. It does not conduct electricity when in solid form but conducts when molten. What is the most likely structure of the substance? Explain.
Date posted: March 28, 2019. Answers (1)
- Study the diagrams below.(Solved)
Study the diagrams below.
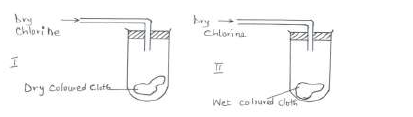
(a) State the observations made at I and II.
(b) Write the equation to show the reactions at II if dry sulphur (IV) oxide was used in place of dry chlorine.
Date posted: March 28, 2019. Answers (1)
- The table below shows elements W, X, Y and Z and their atomic numbers. The letters are not the actual symbols of the elements. Use...(Solved)
The table below shows elements W, X, Y and Z and their atomic numbers. The letters are not the actual symbols of the elements. Use the letters to answer the questions that follow.
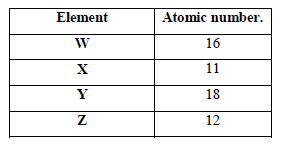
(a) Select an element which forms
(i) Anions.
(ii) An insoluble carbonate
(b) Which element has the largest atomic radius
Date posted: March 28, 2019. Answers (1)
- Briefly explain the following
(a) Atomic radii of alkaline earth metals are smaller than those of the corresponding alkali metals in the same period.
(b) Melting point...(Solved)
Briefly explain the following
(a) Atomic radii of alkaline earth metals are smaller than those of the corresponding alkali metals in the same period.
(b) Melting point of halogens increase down the group.
(c) Helium is a better gas for use in weather research balloons than hydrogen.
Date posted: March 28, 2019. Answers (1)
- An element E has relative atomic mass of 69.39. Given that the element has two isotopes of atomic masses 62.35 and 70.45, calculate the relative...(Solved)
An element E has relative atomic mass of 69.39. Given that the element has two isotopes of atomic masses 62.35 and 70.45, calculate the relative abundance of each of the isotopes.
Date posted: March 28, 2019. Answers (1)
- Study the diagram below and answer the questions that follow.
(a) Identify gas Z.
(b) Write the chemical equation for the reaction which produces gas Z
(c)...(Solved)
Study the diagram below and answer the questions that follow.
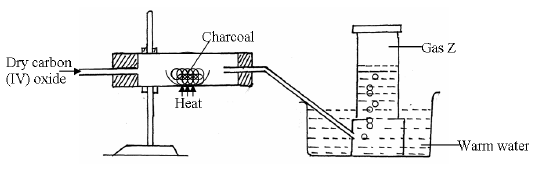
(a) Identify gas Z.
(b) Write the chemical equation for the reaction which produces gas Z
(c) State why the above experiment should be carried out in a fume chamber.
Date posted: March 28, 2019. Answers (1)
- Study the scheme below hence answer the questions that follow.(Solved)
Study the scheme below hence answer the questions that follow.
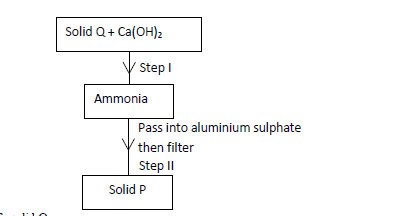
(a) Identify solid Q.
(b) Write an ionic equation for the reaction in Step II.
(c) State the condition necessary for Step I.
Date posted: March 28, 2019. Answers (1)
- Name the method of separation that can most suitably be used to separate the following mixtures.
(a) Gasoline from petroleum
(b) Benzoic acid and potassium carbonate
(c) Oil...(Solved)
Name the method of separation that can most suitably be used to separate the following mixtures.
(a) Gasoline from petroleum
(b) Benzoic acid and potassium carbonate
(c) Oil from cashew nuts
Date posted: March 28, 2019. Answers (1)
- Study the set-up below and answer the questions that follow.
After sometimes, the cotton wools X, Y and Z changed color in turn.
(a) What were the...(Solved)
Study the set-up below and answer the questions that follow.
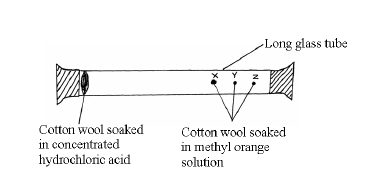
After sometimes, the cotton wools X, Y and Z changed color in turn.
(a) What were the colour changes?
(b) Which cotton wool changed colour first?
(c) Explain why the cotton wools did not change colour at the same time.
Date posted: March 28, 2019. Answers (1)
- A current of 2.5A was passed through a cell containing N2+ ions for 25 minutes. The mass of the cathode increased by 0.36g.Determine the R.A.M...(Solved)
A current of 2.5A was passed through a cell containing N2+ ions for 25 minutes. The mass of the cathode increased by 0.36g.Determine the R.A.M of N. (F = 9.65 x 104Cmolˉ¹).
Date posted: March 28, 2019. Answers (1)
- The diagram below represents a set-up of apparatus used to investigate the effect of electric current on lead (II) bromide.(Solved)
The diagram below represents a set-up of apparatus used to investigate the effect of electric current on lead (II) bromide.
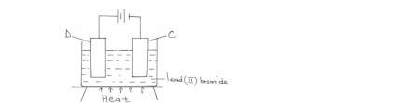
Describe what is observed at electrode C.
Date posted: March 28, 2019. Answers (1)