(a) II
(b) Non-biodegradable hence leads to water pollution.
(c) (RCOO-)2Mg2+.
sharon kalunda answered the question on March 28, 2019 at 14:01
- When one mole of hydrocarbon M was completely burned in air, four moles of carbon (IV) oxide and three moles of water vapour were formed.
a)...(Solved)
When one mole of hydrocarbon M was completely burned in air, four moles of carbon (IV) oxide and three moles of water vapour were formed.
a) Work out the formula of the hydrocarbon.
b) Draw and name two structural formula of hydrocarbon M.
Date posted: March 28, 2019. Answers (1)
- The diagram below represents a section of the hydrochloric acid manufacturing plant.(Solved)
The diagram below represents a section of the hydrochloric acid manufacturing plant.

(a) Name Y.
(b) State the role played by glass beads.
(c) Chlorine reacts with hydrogen sulphide gas according the equation shown below:

From the equation identify the oxidizing agent.
Date posted: March 28, 2019. Answers (1)
- Magnesium hydroxide is the active reagent in antacid tablets.
(i) Explain the effect of chewing the antacid tablets instead of swallowing the whole tablet.
(ii)Starting from aluminium...(Solved)
Magnesium hydroxide is the active reagent in antacid tablets.
(i) Explain the effect of chewing the antacid tablets instead of swallowing the whole tablet.
(ii)Starting from aluminium sulphate, describe how you would obtain aluminium hydroxide
Date posted: March 28, 2019. Answers (1)
- a) Explain why hydrogen gas has been replaced by helium in filling aeroplane tyres.
b)Hydrogen gas is bubbled through oil in presence of a catalyst to...(Solved)
a) Explain why hydrogen gas has been replaced by helium in filling aeroplane tyres.
b)Hydrogen gas is bubbled through oil in presence of a catalyst to yield fats.
(i) Name a suitable catalyst used in the above process.
(ii) What name is given to the above process.
Date posted: March 28, 2019. Answers (1)
- Potassium has two isotopes as shown below;(Solved)
Potassium has two isotopes as shown below;
isotope 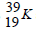 and a radioactive
and a radioactive 
(a) State how the two isotopes differ.
(b) The half-life of  is 1.3 x 109 years. Determine how long it would take for 4g of the isotope to decay to 1g.
is 1.3 x 109 years. Determine how long it would take for 4g of the isotope to decay to 1g.
c)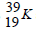 undergoes beta decay to form an isotope of calcium. Write the equation for this decay.
undergoes beta decay to form an isotope of calcium. Write the equation for this decay.
Date posted: March 28, 2019. Answers (1)
- 4. 9 g a tribasic acid was dissolved in water and the solution made up to 500cm3. If the molarity of the hydrogen ions in...(Solved)
4. 9 g a tribasic acid was dissolved in water and the solution made up to 500cm3. If the molarity of the hydrogen ions in the solution is 0.3 molar calculate the relative molecular mass of the acid.
Date posted: March 28, 2019. Answers (1)
- The diagram below represents pipes used in Frasch pump for the extraction of sulpur
Name the substance that pass through the following tubes and the direction...(Solved)
The diagram below represents pipes used in Frasch pump for the extraction of sulpur

Name the substance that pass through the following tubes and the direction which they flow.
1.
2.
3.
Date posted: March 28, 2019. Answers (1)
- 12.0 cm3 of methane and 48cm3 of oxygen were exploded together. The final volume measured under the original conditions was 36.0 cm3 neglecting the water...(Solved)
12.0 cm3 of methane and 48cm3 of oxygen were exploded together. The final volume measured under the original conditions was 36.0 cm3 neglecting the water formed. 24.0cm3 of this was unused oxygen. Show the ratio of reacting volume of the gases referred to and gaseous products formed.
Date posted: March 28, 2019. Answers (1)
- The molecular formula of compound T is C3H8O. T reacts with acidified potassium manganate (VII) to form another compound U whose formula C3H6O2. T also...(Solved)
The molecular formula of compound T is C3H8O. T reacts with acidified potassium manganate (VII) to form another compound U whose formula C3H6O2. T also reacts with sodium metal to produce hydrogen gas and T is neutral to litmus.
(a) Suggest the homologous series to which T belongs.
(b) Name the type of reaction leading to the formation of U in the reaction described above.
(c) Write the structural formula of U.
Date posted: March 28, 2019. Answers (1)
- Element X contains isotopes with mass number 16 and 18 respectively existing in the ratio
1: 3. Calculate the relative atomic mass of X.(Solved)
Element X contains isotopes with mass number 16 and 18 respectively existing in the ratio
1: 3. Calculate the relative atomic mass of X.
Date posted: March 28, 2019. Answers (1)
- What volume of methane would remain if a burner containing 40cm³ of methane burns in 40cm³ of enclosed air
(assuming that oxygen is 20% of air)?(Solved)
What volume of methane would remain if a burner containing 40cm³ of methane burns in 40cm³ of enclosed air
(assuming that oxygen is 20% of air)?
Date posted: March 28, 2019. Answers (1)
- Two samples of a similar substance from different containers were investigated. The curve below represents the variation of temperature with time when heated separately.
a) Explain...(Solved)
Two samples of a similar substance from different containers were investigated. The curve below represents the variation of temperature with time when heated separately.
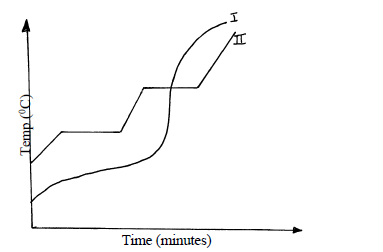
a) Explain the variation in the curves.
Sample I
Sample II
b) Common salt is sprinkled on roads during winter in temperate countries.
Date posted: March 28, 2019. Answers (1)
- A compound has empirical formula C3H6O and relative formula mass of 116. Determine it’s molecular formula.(C = 12, H = 1, O = 16).(Solved)
A compound has empirical formula C3H6O and relative formula mass of 116. Determine it’s molecular formula.(C = 12, H = 1, O = 16).
Date posted: March 28, 2019. Answers (1)
- Element A and B with atomic numbers 12 and 17 respectively react together.
(a) Write the electronic configurations of each
A:
...(Solved)
Element A and B with atomic numbers 12 and 17 respectively react together.
(a) Write the electronic configurations of each
A:
B:
(b) Write the formula of the compound formed between A and B.
Date posted: March 28, 2019. Answers (1)
- Explain why it is not advisable to use potassium chloride as salt bridge in the cell shown below.(Solved)
Explain why it is not advisable to use potassium chloride as salt bridge in the cell shown below.
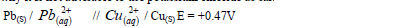
Date posted: March 28, 2019. Answers (1)
- Aqueous sodium sulphate was electrolysed using platinum. Name the products at each electrode.
I Cathode
IIAnode(Solved)
Aqueous sodium sulphate was electrolysed using platinum. Name the products at each electrode.
I Cathode
IIAnode
Date posted: March 28, 2019. Answers (1)
- When bromine gas reacts with aqueous sodium hydroxide an equilibrium is established as shown below.(Solved)
When bromine gas reacts with aqueous sodium hydroxide an equilibrium is established as shown below.
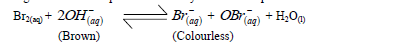
What observation would be made if a few drops of dilute sulphuric (VI) were added to the equilibrium mixture? Explain.
Date posted: March 28, 2019. Answers (1)
- Butane burns in air according to the equation shown below.
2C4H10(g) + 13O2(g) ----> 8CO2(g) + 10H2O(l)
What volume of butane must be burnt in oxygen to...(Solved)
Butane burns in air according to the equation shown below.
2C4H10(g) + 13O2(g) ----> 8CO2(g) + 10H2O(l)
What volume of butane must be burnt in oxygen to give 11g of CO2 at r.t.p?
(Molar gas volume at r.t.p = 24.0L; C = 120; O = 16.0; H = 1.0 )
Date posted: March 28, 2019. Answers (1)
- Study the cycle below hence answer the questions that follow.(Solved)
Study the cycle below hence answer the questions that follow.
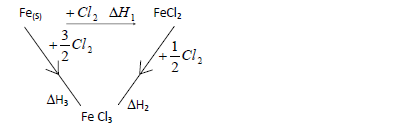
(a) What is (change)H3?
(b) Show the relationship connecting (change)H1,(change)H2 and (change)H3
Date posted: March 28, 2019. Answers (1)
- A gas of known mass occupies 200cm3 at 250C and 101325 Pa pressure. What volume would it occupy
at -230C and 1000 Pa pressure?(Solved)
A gas of known mass occupies 200cm3 at 250C and 101325 Pa pressure. What volume would it occupy
at -230C and 1000 Pa pressure?
Date posted: March 28, 2019. Answers (1)