I) No rusting. Zinc is above iron in the reactivity series
(II) Rusting occurs. Iron is more reactive than copper
sharon kalunda answered the question on March 28, 2019 at 14:35
- Name the chief ore of iron and write its formula(Solved)
Name the chief ore of iron and write its formula
Date posted: March 28, 2019. Answers (1)
- Name the following processes;
a) When anhydrous calcium chloride is left in an open beaker overnight a solution was formed.
b) When sodium carbonate decahydrate crystals...(Solved)
Name the following processes;
a) When anhydrous calcium chloride is left in an open beaker overnight a solution was formed.
b) When sodium carbonate decahydrate crystals are left in an open beaker for some days it turned into a powder.
Date posted: March 28, 2019. Answers (1)
- Name the following organic compounds.(Solved)
Name the following organic compounds.

Date posted: March 28, 2019. Answers (1)
- The diagram below represents a method of separation used to separate two liquids A and B. use it to answer the questions that follow(Solved)
The diagram below represents a method of separation used to separate two liquids A and B. use it to answer the questions that follow

a) Name two properties that makes it possible for the two liquids to be separated.
b) Give one alternative method that may be used to separate the two liquids.
Date posted: March 28, 2019. Answers (1)
- 60cm³ of oxygen gas diffused through a porous hole in 50 seconds. How long will it take 80cm³ of sulphur (IV) oxide to diffuse through...(Solved)
60cm³ of oxygen gas diffused through a porous hole in 50 seconds. How long will it take 80cm³ of sulphur (IV) oxide to diffuse through the same hole under the same conditions (S = 32, O = 16).
Date posted: March 28, 2019. Answers (1)
- Starting with solid lead (II) carbonate, briefly describe how a sample of lead (II) chloride can be prepared.(Solved)
Starting with solid lead (II) carbonate, briefly describe how a sample of lead (II) chloride can be prepared.
Date posted: March 28, 2019. Answers (1)
- The formulae below belong to 2 cleansing agents.(Solved)
The formulae below belong to 2 cleansing agents.

(a) Which of the two cleaning agents would lather readily with hard water?
(b) State one disadvantage of the continued use of cleansing agent II.
(c) Write the formula of the compound formed when cleansing agent I is used with water containing Mg2+ ions.
Date posted: March 28, 2019. Answers (1)
- When one mole of hydrocarbon M was completely burned in air, four moles of carbon (IV) oxide and three moles of water vapour were formed.
a)...(Solved)
When one mole of hydrocarbon M was completely burned in air, four moles of carbon (IV) oxide and three moles of water vapour were formed.
a) Work out the formula of the hydrocarbon.
b) Draw and name two structural formula of hydrocarbon M.
Date posted: March 28, 2019. Answers (1)
- The diagram below represents a section of the hydrochloric acid manufacturing plant.(Solved)
The diagram below represents a section of the hydrochloric acid manufacturing plant.

(a) Name Y.
(b) State the role played by glass beads.
(c) Chlorine reacts with hydrogen sulphide gas according the equation shown below:

From the equation identify the oxidizing agent.
Date posted: March 28, 2019. Answers (1)
- Magnesium hydroxide is the active reagent in antacid tablets.
(i) Explain the effect of chewing the antacid tablets instead of swallowing the whole tablet.
(ii)Starting from aluminium...(Solved)
Magnesium hydroxide is the active reagent in antacid tablets.
(i) Explain the effect of chewing the antacid tablets instead of swallowing the whole tablet.
(ii)Starting from aluminium sulphate, describe how you would obtain aluminium hydroxide
Date posted: March 28, 2019. Answers (1)
- a) Explain why hydrogen gas has been replaced by helium in filling aeroplane tyres.
b)Hydrogen gas is bubbled through oil in presence of a catalyst to...(Solved)
a) Explain why hydrogen gas has been replaced by helium in filling aeroplane tyres.
b)Hydrogen gas is bubbled through oil in presence of a catalyst to yield fats.
(i) Name a suitable catalyst used in the above process.
(ii) What name is given to the above process.
Date posted: March 28, 2019. Answers (1)
- Potassium has two isotopes as shown below;(Solved)
Potassium has two isotopes as shown below;
isotope 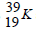 and a radioactive
and a radioactive 
(a) State how the two isotopes differ.
(b) The half-life of  is 1.3 x 109 years. Determine how long it would take for 4g of the isotope to decay to 1g.
is 1.3 x 109 years. Determine how long it would take for 4g of the isotope to decay to 1g.
c)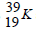 undergoes beta decay to form an isotope of calcium. Write the equation for this decay.
undergoes beta decay to form an isotope of calcium. Write the equation for this decay.
Date posted: March 28, 2019. Answers (1)
- 4. 9 g a tribasic acid was dissolved in water and the solution made up to 500cm3. If the molarity of the hydrogen ions in...(Solved)
4. 9 g a tribasic acid was dissolved in water and the solution made up to 500cm3. If the molarity of the hydrogen ions in the solution is 0.3 molar calculate the relative molecular mass of the acid.
Date posted: March 28, 2019. Answers (1)
- The diagram below represents pipes used in Frasch pump for the extraction of sulpur
Name the substance that pass through the following tubes and the direction...(Solved)
The diagram below represents pipes used in Frasch pump for the extraction of sulpur

Name the substance that pass through the following tubes and the direction which they flow.
1.
2.
3.
Date posted: March 28, 2019. Answers (1)
- 12.0 cm3 of methane and 48cm3 of oxygen were exploded together. The final volume measured under the original conditions was 36.0 cm3 neglecting the water...(Solved)
12.0 cm3 of methane and 48cm3 of oxygen were exploded together. The final volume measured under the original conditions was 36.0 cm3 neglecting the water formed. 24.0cm3 of this was unused oxygen. Show the ratio of reacting volume of the gases referred to and gaseous products formed.
Date posted: March 28, 2019. Answers (1)
- The molecular formula of compound T is C3H8O. T reacts with acidified potassium manganate (VII) to form another compound U whose formula C3H6O2. T also...(Solved)
The molecular formula of compound T is C3H8O. T reacts with acidified potassium manganate (VII) to form another compound U whose formula C3H6O2. T also reacts with sodium metal to produce hydrogen gas and T is neutral to litmus.
(a) Suggest the homologous series to which T belongs.
(b) Name the type of reaction leading to the formation of U in the reaction described above.
(c) Write the structural formula of U.
Date posted: March 28, 2019. Answers (1)
- Element X contains isotopes with mass number 16 and 18 respectively existing in the ratio
1: 3. Calculate the relative atomic mass of X.(Solved)
Element X contains isotopes with mass number 16 and 18 respectively existing in the ratio
1: 3. Calculate the relative atomic mass of X.
Date posted: March 28, 2019. Answers (1)
- What volume of methane would remain if a burner containing 40cm³ of methane burns in 40cm³ of enclosed air
(assuming that oxygen is 20% of air)?(Solved)
What volume of methane would remain if a burner containing 40cm³ of methane burns in 40cm³ of enclosed air
(assuming that oxygen is 20% of air)?
Date posted: March 28, 2019. Answers (1)
- Two samples of a similar substance from different containers were investigated. The curve below represents the variation of temperature with time when heated separately.
a) Explain...(Solved)
Two samples of a similar substance from different containers were investigated. The curve below represents the variation of temperature with time when heated separately.
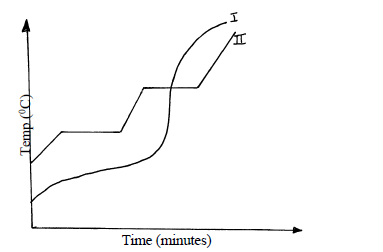
a) Explain the variation in the curves.
Sample I
Sample II
b) Common salt is sprinkled on roads during winter in temperate countries.
Date posted: March 28, 2019. Answers (1)
- A compound has empirical formula C3H6O and relative formula mass of 116. Determine it’s molecular formula.(C = 12, H = 1, O = 16).(Solved)
A compound has empirical formula C3H6O and relative formula mass of 116. Determine it’s molecular formula.(C = 12, H = 1, O = 16).
Date posted: March 28, 2019. Answers (1)