a) Carbon (IV) Oxide // CO2 / Carbon dioxide
b) Filtration
c) 2NaHCO3(s) ---> Na2CO3(s) + H2O(l) + CO2(g)
d) Reacted with Calcium hydroxide to generate ammonia
Kavungya answered the question on March 29, 2019 at 05:47
-
Using dots (.) and crosses (x) to represent electrons, show the bonding in hydroxonium ion (H=1 O=8).
(Solved)
Using dots (.) and crosses (x) to represent electrons, show the bonding in hydroxonium ion (H=1 O=8).
Date posted:
March 29, 2019
.
Answers (1)
-
In an experiment, two pieces of iron sheets were wrapped in each case with zinc and copper metal sheets as shown below.They were left in...
(Solved)
In an experiment, two pieces of iron sheets were wrapped in each case with zinc and copper metal sheets as shown below.They were left in the open for some months.

State and explain the observations made in the experiments; (I), (II)
Date posted:
March 28, 2019
.
Answers (1)
-
Name the following processes;
a) When anhydrous calcium chloride is left in an open beaker overnight a solution was formed.
b) When sodium carbonate decahydrate crystals...
(Solved)
Name the following processes;
a) When anhydrous calcium chloride is left in an open beaker overnight a solution was formed.
b) When sodium carbonate decahydrate crystals are left in an open beaker for some days it turned into a powder.
Date posted:
March 28, 2019
.
Answers (1)
-
60cm³ of oxygen gas diffused through a porous hole in 50 seconds. How long will it take 80cm³ of sulphur (IV) oxide to diffuse through...
(Solved)
60cm³ of oxygen gas diffused through a porous hole in 50 seconds. How long will it take 80cm³ of sulphur (IV) oxide to diffuse through the same hole under the same conditions (S = 32, O = 16).
Date posted:
March 28, 2019
.
Answers (1)
-
Starting with solid lead (II) carbonate, briefly describe how a sample of lead (II) chloride can be prepared.
(Solved)
Starting with solid lead (II) carbonate, briefly describe how a sample of lead (II) chloride can be prepared.
Date posted:
March 28, 2019
.
Answers (1)
-
a) Explain why hydrogen gas has been replaced by helium in filling aeroplane tyres.
b)Hydrogen gas is bubbled through oil in presence of a catalyst to...
(Solved)
a) Explain why hydrogen gas has been replaced by helium in filling aeroplane tyres.
b)Hydrogen gas is bubbled through oil in presence of a catalyst to yield fats.
(i) Name a suitable catalyst used in the above process.
(ii) What name is given to the above process.
Date posted:
March 28, 2019
.
Answers (1)
-
4. 9 g a tribasic acid was dissolved in water and the solution made up to 500cm3. If the molarity of the hydrogen ions in...
(Solved)
4. 9 g a tribasic acid was dissolved in water and the solution made up to 500cm3. If the molarity of the hydrogen ions in the solution is 0.3 molar calculate the relative molecular mass of the acid.
Date posted:
March 28, 2019
.
Answers (1)
-
The diagram below represents pipes used in Frasch pump for the extraction of sulpur
Name the substance that pass through the following tubes and the direction...
(Solved)
The diagram below represents pipes used in Frasch pump for the extraction of sulpur

Name the substance that pass through the following tubes and the direction which they flow.
1.
2.
3.
Date posted:
March 28, 2019
.
Answers (1)
-
12.0 cm3 of methane and 48cm3 of oxygen were exploded together. The final volume measured under the original conditions was 36.0 cm3 neglecting the water...
(Solved)
12.0 cm3 of methane and 48cm3 of oxygen were exploded together. The final volume measured under the original conditions was 36.0 cm3 neglecting the water formed. 24.0cm3 of this was unused oxygen. Show the ratio of reacting volume of the gases referred to and gaseous products formed.
Date posted:
March 28, 2019
.
Answers (1)
-
Element X contains isotopes with mass number 16 and 18 respectively existing in the ratio
1: 3. Calculate the relative atomic mass of X.
(Solved)
Element X contains isotopes with mass number 16 and 18 respectively existing in the ratio
1: 3. Calculate the relative atomic mass of X.
Date posted:
March 28, 2019
.
Answers (1)
-
What volume of methane would remain if a burner containing 40cm³ of methane burns in 40cm³ of enclosed air
(assuming that oxygen is 20% of air)?
(Solved)
What volume of methane would remain if a burner containing 40cm³ of methane burns in 40cm³ of enclosed air
(assuming that oxygen is 20% of air)?
Date posted:
March 28, 2019
.
Answers (1)
-
A compound has empirical formula C3H6O and relative formula mass of 116. Determine it’s molecular formula.(C = 12, H = 1, O = 16).
(Solved)
A compound has empirical formula C3H6O and relative formula mass of 116. Determine it’s molecular formula.(C = 12, H = 1, O = 16).
Date posted:
March 28, 2019
.
Answers (1)
-
The scheme below shows some reaction of salt. Study it and answer questions that follow.
(Solved)
The scheme below shows some reaction of salt. Study it and answer questions that follow.
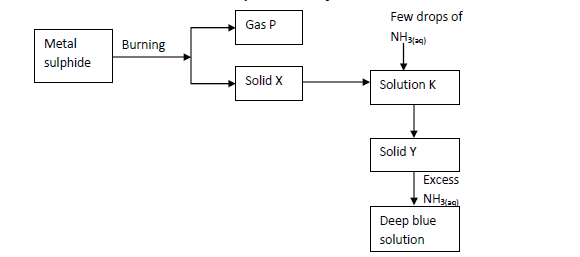
(i) Write an equation for the reaction to show formation of gas P and solid X.
(ii) Give the name and formula of the complex ion responsible for the deep blue colour in the solution.
Date posted:
March 28, 2019
.
Answers (1)
-
Explain why potassium chloride conducts electricity in both the molten state and in aqueous solution whereas hydrogen chloride conducts electricity only in aqueous solution and...
(Solved)
Explain why potassium chloride conducts electricity in both the molten state and in aqueous solution whereas hydrogen chloride conducts electricity only in aqueous solution and not in gaseous state.
Date posted:
March 28, 2019
.
Answers (1)
-
An element E has relative atomic mass of 69.39. Given that the element has two isotopes of atomic masses 62.35 and 70.45, calculate the relative...
(Solved)
An element E has relative atomic mass of 69.39. Given that the element has two isotopes of atomic masses 62.35 and 70.45, calculate the relative abundance of each of the isotopes.
Date posted:
March 28, 2019
.
Answers (1)
-
Name the method of separation that can most suitably be used to separate the following mixtures.
(a) Gasoline from petroleum
(b) Benzoic acid and potassium carbonate
(c) Oil...
(Solved)
Name the method of separation that can most suitably be used to separate the following mixtures.
(a) Gasoline from petroleum
(b) Benzoic acid and potassium carbonate
(c) Oil from cashew nuts
Date posted:
March 28, 2019
.
Answers (1)
-
25.0cm3 of 0.12M potassium hydroxide solution required 30.0cm3 of a solution of a dibasic acid (H2Y) for complete neutralization. The acid contained 3.15g per 500cm3...
(Solved)
25.0cm3 of 0.12M potassium hydroxide solution required 30.0cm3 of a solution of a dibasic acid (H2Y) for complete neutralization. The acid contained 3.15g per 500cm3 solution.
Calculate:
(a) The molarity of the acid solution
(b) The relative formula mass of the acid.
Date posted:
March 28, 2019
.
Answers (1)
-
A Student in form four placed a thermometer in molten naphthalene at 850C and recorded the temperature and time until the naphthalene solidified. From the...
(Solved)
A Student in form four placed a thermometer in molten naphthalene at 850C and recorded the temperature and time until the naphthalene solidified. From the values obtained, the figure below was drawn.
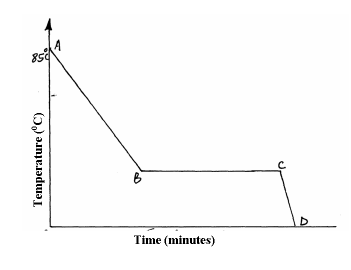
(a) What name is given to such a figure?
(b) Which part of the figure represents the change of state of naphthalene?
(c)In terms of kinetic theory. Explain what happens to molecules along AB.
Date posted:
March 28, 2019
.
Answers (1)
-
The table below shows information about three solid substances A, B and C. Study it and answer the question that follow.
Describe how you will separate...
(Solved)
The table below shows information about three solid substances A, B and C. Study it and answer the question that follow.
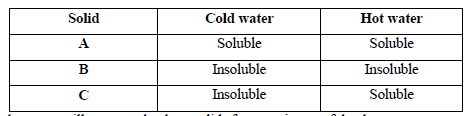
Describe how you will separate the three solids from a mixture of the three.
Date posted:
March 28, 2019
.
Answers (1)
-
Calculate the volume of carbon (IV) oxide that would be produced if 15g of calcium carbonate reacted with 100cm³ of 2.0M hydrochloric acid (C =...
(Solved)
Calculate the volume of carbon (IV) oxide that would be produced if 15g of calcium carbonate reacted with 100cm³ of 2.0M hydrochloric acid (C = 12.0, O = 16.0, Ca = 40.0) molar gas volume = 24000cm³
Date posted:
March 28, 2019
.
Answers (1)