- Study the information below and answer the questions that follow.(Solved)
Study the information below and answer the questions that follow.
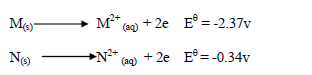
(i) Which of the two is a stronger oxidizing agent?
(ii) Write the overall redox reaction.
(iii) Calculate the E.M.F of the cell.
Date posted: April 1, 2019. Answers (1)
- A compound C3H8O reacts with sodium metal, forming a basic solution and a colourless gas.(Solved)
A compound C3H8O reacts with sodium metal, forming a basic solution and a colourless gas.
I) To which class of organic compounds does it belong to.
II) Identify the
(i) Basic solution
(ii) Colourless gas
Date posted: April 1, 2019. Answers (1)
- 57.2g of hydrated sodium carbonate (Na2CO3.XH2O) were dissolved in water and the solution made to one litre. 20cm3 of 0.5M HCl recated with 25cm3 of...(Solved)
57.2g of hydrated sodium carbonate (Na2CO3.XH2O) were dissolved in water and the solution made to one litre. 20cm3 of 0.5M HCl recated with 25cm3 of the hydrated sodium carbonate solution. Determine the value of X. (Na = 23, C = 12, O = 16)
Date posted: April 1, 2019. Answers (1)
- A student tried to prepare aluminium carbonate by mixing a solution of aluminium chloride and a solution of sodium carbonate but did not succeed. Explain.(Solved)
A student tried to prepare aluminium carbonate by mixing a solution of aluminium chloride and a solution of sodium carbonate but did not succeed. Explain.
Date posted: April 1, 2019. Answers (1)
- Phosphorus has an atomic number of 15. State the type of bond formed when phosphorous combines with bromine.(Solved)
Phosphorus has an atomic number of 15. State the type of bond formed when phosphorous combines with bromine.
Date posted: April 1, 2019. Answers (1)
- Using oxidation numbers identify the oxidizing agent in the reaction below.(Solved)
Using oxidation numbers identify the oxidizing agent in the reaction below.

Date posted: April 1, 2019. Answers (1)
- Would copper be suitable to prevent rusting in iron? Explain(Solved)
Would copper be suitable to prevent rusting in iron? Explain.
Date posted: April 1, 2019. Answers (1)
- Filtration is usually carried out in the purification of water. Which other process would be required to obtain water for drinking?(Solved)
Filtration is usually carried out in the purification of water. Which other process would be required to obtain water for drinking?
Date posted: April 1, 2019. Answers (1)
- A gas occupies 250cm3 at 1000C at a pressure of 760mmHg. Calculate the volume
of the gas at 2000C at 760mmHg.(Solved)
A gas occupies 250cm3 at 1000C at a pressure of 760mmHg. Calculate the volume
of the gas at 2000C at 760mmHg.
Date posted: April 1, 2019. Answers (1)
- a)A hydrocarbon decolourises both bromine gas and acidified potassium permanganate.Name and draw the structure of the fourth member of the series to which the hydrocarbon...(Solved)
a)A hydrocarbon decolourises both bromine gas and acidified potassium permanganate.Name and draw the structure of the fourth member of the series to which the hydrocarbon belongs.
b)To which homologous series does (a) above belong to.
Date posted: April 1, 2019. Answers (1)
- The formula given below represents a portion of a polymer.(Solved)
The formula given below represents a portion of a polymer.

(i) Give the name of the monomer.
(ii) State one disadvantage of continued use of the polymer.
(iii) State one use of the polymer.
Date posted: April 1, 2019. Answers (1)
- The table below shows the formulae of the chlorides of P, Q, R and S ( not the actual symbols )(Solved)
The table below shows the formulae of the chlorides of P, Q, R and S ( not the actual symbols )

(i) State the group to which the elements belong.
P
Q
R
S
(ii) Which of the chlorides would conduct electricity when dissolved in water . Explain.
Date posted: April 1, 2019. Answers (1)
- The equilibrium reaction of phenolphthalein indicator in water may be represented as follows.(Solved)
The equilibrium reaction of phenolphthalein indicator in water may be represented as follows.

State and explain the observations made when an acid is added to the equilibrium
mixture.
Date posted: April 1, 2019. Answers (1)
- Study the chart below and answer the questions that follow.(Solved)
Study the chart below and answer the questions that follow.

(i) Identify metal ion present in solution M.
(ii) Give the formula of the complex ion in
I) Solution P
II) Solution N
Date posted: April 1, 2019. Answers (1)
- Draw the isomers of the compound C3H7Cl and give their systematic names.(Solved)
Draw the isomers of the compound C3H7Cl and give their systematic names.
Date posted: April 1, 2019. Answers (1)
- What do you understand by the term isomers ?(Solved)
What do you understand by the term isomers ?
Date posted: April 1, 2019. Answers (1)
- Calculate the mass of calcium carbonate that would react completely with 20cm3 of 0.2M hydrochloric acid (Ca = 40.0, C = 12.0, O = 16.0)(Solved)
Calculate the mass of calcium carbonate that would react completely with 20cm 3 of 0.2M hydrochloric acid (Ca = 40.0, C = 12.0, O = 16.0)
Date posted: April 1, 2019. Answers (1)
- The following graph represents a thermochemical process(Solved)
The following graph represents a thermochemical process

(a) What does Y represent.
(b) Is the overall reaction exothermic or endothermic. Explain your answer.
Date posted: April 1, 2019. Answers (1)
- The atomic numbers of elements A and B is 13 and 8 respectively.
(i) Write electronic arrangements of the atoms of A and B.
(ii) Name the...(Solved)
The atomic numbers of elements A and B is 13 and 8 respectively.
(i) Write electronic arrangements of the atoms of A and B.
(ii) Name the type of bond formed by the elements A and
Date posted: April 1, 2019. Answers (1)
- Why is water not used to put off oil fires?(Solved)
Why is water not used to put off oil fires?
Date posted: March 29, 2019. Answers (1)