- A form four student prepared potassium sulphite solution and divided it into two portions.The first portion gave a white precipitate when reacted with barium nitrate....(Solved)
A form four student prepared potassium sulphite solution and divided it into two portions.The first portion gave a white precipitate when reacted with barium nitrate. On addition of dilute hydrochloric acid, the white precipitate disappeared.
(a) Write the formula of the compound which formed as the white precipitate.
(b) Write the equation for the reaction between dilute hydrochloric acid and the
compound whose formula is written in (a) above.
Date posted: April 1, 2019. Answers (1)
- A compound whose structure is shown below is found in a detergent, used in our day to day activities.(Solved)
A compound whose structure is shown below is found in a detergent, used in our day to day activities.

With reference to the structure, explain how the detergent removes grease during washing.
Date posted: April 1, 2019. Answers (1)
- Ammonia can be converted to nitrogen (II) Oxide as shown in the equation below in a research centre.(Solved)
Ammonia can be converted to nitrogen (II) Oxide as shown in the equation below in a research centre.


(a) Explain how an increase in temperature would affect the yield of nitrogen (II) oxide.
(b) On the energy level diagram above sketch, the energy level diagram that would be
obtained if the reaction is carried out in the presence of platinum catalyst.
Date posted: April 1, 2019. Answers (1)
- The diagram below represents the extraction of sulphur by frasch process in Louisiana U.S.A.(Solved)
The diagram below represents the extraction of sulphur by frasch process in Louisiana U.S.A.

(a) Name the substance that passes through tube:-
I
II
(b) What is the purpose of hot compressed air in this process ?
Date posted: April 1, 2019. Answers (1)
- The following are the standard electrode potential for some electrodes.(Solved)
The following are the standard electrode potential for some electrodes

(i) Which is the strongest reducing agent. Explain.
(ii) Write the cell representation for the electrochemical cell obtained by combining the half cells B and D.
(iii) Calculate the e.m.f of the cell in (ii) above.
(iv) Give one use of electrochemical cells.
Date posted: April 1, 2019. Answers (1)
- Give the differences between esterification and neutralisation.(Solved)
Give the differences between esterification and neutralisation.
Date posted: April 1, 2019. Answers (1)
- (i) Name the type of reaction illustrated by the above reaction. (ii) Name the catalyst used in the reaction (Solved)

(i) Name the type of reaction illustrated by the above reaction.
(ii) Name the catalyst used in the reaction above.
(iii) Write an equation for the reaction between P and sodium hydroxide solution.
(iv) Name the type of reaction in (III) above.
Date posted: April 1, 2019. Answers (1)
- Study the flow chart below and answer the questions that follow.(Solved)
Study the flow chart below and answer the questions that follow.

(i) Identify
A
B
C
D
(ii) Write a chemical equation of a reaction in step I and II leading to formation of the
first member in B and alkyne series.
Step I
Step II
Date posted: April 1, 2019. Answers (1)
- Give the IUPAC names of the following(Solved)
Give the IUPAC names of the following

Date posted: April 1, 2019. Answers (1)
- The table below gives elements represented by letters U V W X Y Z and their atomic numbers.(Solved)
The table below gives elements represented by letters U V W X Y Z and their atomic numbers.

Use the information to answer the questions below.
(a) In which period do these elements belong ? Give a reason.
(b) How does the atomic radius of W and Y compare ? Explain.
(c ) Give the formula of the compound that could be formed between V and X.
(d) What type of bonding will be present in a compound formed between V and Z. Explain.
(e) Arrange the species U, U- and U+ in increasing order of size.
(f) Which of the ions Y2+ and Y2- is the most stable ? Explain.
(g) Give the formula of
(i) An acidic oxide formed when one of the elements in the table is heated in air.
(ii) A basic oxide formed when one of the elements in the table is heated in air.
Date posted: April 1, 2019. Answers (1)
- Study the diagram below and answer the questions that follows.(Solved)
Study the diagram below and answer the questions that follows.

Calculate the value of y. (H = 1.0, Cl = 35.5, n = 14.0)
Date posted: April 1, 2019. Answers (1)
- Uranium – 238 undergoes a radioactive decay according to the equation(Solved)
Uranium – 238 undergoes a radioactive decay according to the equation

(i) Determine the value of
x
y
(ii) Why is it not suitable to store radioactive materials in aluminium containers.
(iii) State one use of radio isotopes.
Date posted: April 1, 2019. Answers (1)
- Bromine reacts with ethane as shown below.(Solved)
Bromine reacts with ethane as shown below.

(a) What condition is necessary for this reaction to occur ?
(b) Identify the bonds which are broken and those that are formed.
Bonds broken
Bonds formed
Date posted: April 1, 2019. Answers (1)
- The formula of the phosphate of Y is Y3(PO4)2. Write the formula of the chloride of Y.(Solved)
The formula of the phosphate of Y is Y3(PO4)2. Write the formula of the chloride of Y.
Date posted: April 1, 2019. Answers (1)
- All the heat given out when 0.6g of an element W were burnt in oxygen was used to heat 500cm3 of water. The temperature of...(Solved)
All the heat given out when 0.6g of an element W were burnt in oxygen was used to heat 500cm3 of water. The temperature of the water rose from 230C to 320C. Calculate the relative atomic mass of element W given that its molar heat of combustion is 380kJ/mol (specific heat capacity of water = 4.2J/g/k, density = 1g cm-3)
Date posted: April 1, 2019. Answers (1)
- Study the information below and answer the questions that follow.(Solved)
Study the information below and answer the questions that follow.
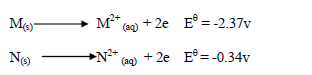
(i) Which of the two is a stronger oxidizing agent?
(ii) Write the overall redox reaction.
(iii) Calculate the E.M.F of the cell.
Date posted: April 1, 2019. Answers (1)
- A compound C3H8O reacts with sodium metal, forming a basic solution and a colourless gas.(Solved)
A compound C3H8O reacts with sodium metal, forming a basic solution and a colourless gas.
I) To which class of organic compounds does it belong to.
II) Identify the
(i) Basic solution
(ii) Colourless gas
Date posted: April 1, 2019. Answers (1)
- 57.2g of hydrated sodium carbonate (Na2CO3.XH2O) were dissolved in water and the solution made to one litre. 20cm3 of 0.5M HCl recated with 25cm3 of...(Solved)
57.2g of hydrated sodium carbonate (Na2CO3.XH2O) were dissolved in water and the solution made to one litre. 20cm3 of 0.5M HCl recated with 25cm3 of the hydrated sodium carbonate solution. Determine the value of X. (Na = 23, C = 12, O = 16)
Date posted: April 1, 2019. Answers (1)
- A student tried to prepare aluminium carbonate by mixing a solution of aluminium chloride and a solution of sodium carbonate but did not succeed. Explain.(Solved)
A student tried to prepare aluminium carbonate by mixing a solution of aluminium chloride and a solution of sodium carbonate but did not succeed. Explain.
Date posted: April 1, 2019. Answers (1)
- Phosphorus has an atomic number of 15. State the type of bond formed when phosphorous combines with bromine.(Solved)
Phosphorus has an atomic number of 15. State the type of bond formed when phosphorous combines with bromine.
Date posted: April 1, 2019. Answers (1)