Heat lost by naphthalene
T = 100-80=20k
H1 = 0.5kg x 2100JK/g/K x 20K = 2100J
Lf = mLf = 0.5kg x 170000J/Kg = 85000J
T = 80 -20 = 60k ,
H2 = 0.5kg x 2100J/Kg/K x 60k = 63000J
Heat lost by aluminium
T = 100 – 20 = 80k
H = 0.4kg x 900J/Kg/K x80k = 28800J
Total heat lost = 169000J + 28800J = 197800J
= 197.8KJ
= 198KJ
sharon kalunda answered the question on April 2, 2019 at 08:23
-
Figure below shows a test tube partially filled with water. An ice wrapped in wire gauze is placed at the bottom of the test-tube. It...
(Solved)
Figure below shows a test tube partially filled with water. An ice wrapped in wire gauze is placed at the bottom of the test-tube. It is then held in the flame of a bunsen burner as shown below
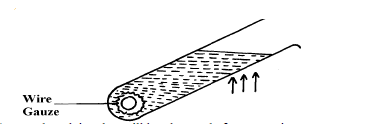
State and explain what will be observed after some time
Date posted:
April 2, 2019
.
Answers (1)
-
The figure below shows two identical thermometers. Thermometer A has a blackened bulb while thermometer B has a silvery bulb. A candle is placed equidistant between...
(Solved)
The figure below shows two identical thermometers. Thermometer A has a blackened bulb while thermometer B has a silvery bulb. A candle is placed equidistant between the two thermometers.
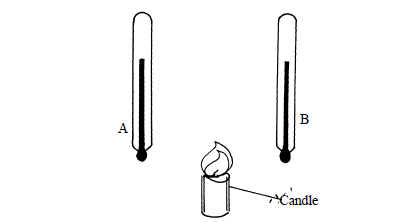
State with a reason the observations made after some time
Date posted:
April 2, 2019
.
Answers (1)
-
Figure below shows a cross-section of a vacuum flask (i) Name the parts labelled A and B on the diagram...
(Solved)
Figure below shows a cross-section of a vacuum flask
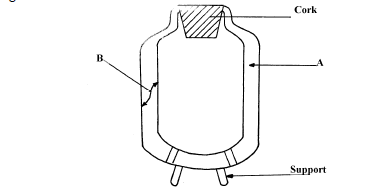
(i) Name the parts labelled A and B on the diagram
(ii) Explain how the heat losses are minimized when hot liquid is poured into the flask
Date posted:
April 2, 2019
.
Answers (1)
-
A jet delivering 0.44g of dry steam per second, at 100oC is directed on to crushed ice at 0.0oC contained in an unlagged copper can...
(Solved)
A jet delivering 0.44g of dry steam per second, at 100oC is directed on to crushed ice at 0.0oC contained in an unlagged copper can which has a hole in the base. 4.44g of water at 0.0oC flow out of the hole per second.
(i) How many joules of heat are given out per second by condensing steam and cooling to 0.0oC of water formed?(Latent heat of vaporization of steam = 2.26 x 106JKg-1,c for water = 4200JKg-1K-1)
(ii) How much heat is taken in per second by the ice which melts?
(iii) Suggest why these amounts above are different
Date posted:
April 2, 2019
.
Answers (1)
-
A block of metal of mass 150g at 100oC is dropped into a logged calorimeter of heat capacity
40J/k containing 100g of water at 25oC. The...
(Solved)
A block of metal of mass 150g at 100oC is dropped into a logged calorimeter of heat capacity
40J/k containing 100g of water at 25oC. The temperature of the resulting mixture is 34oC.
(Specific heat capacity of water = 4200J/KgK)
Determine;-
(i) Heat gained by calorimeter
(ii) Heat gained by water
(iii) Heat lost by the metal block
(iv) Specific heat capacity of the metal block
Date posted:
April 2, 2019
.
Answers (1)
-
A block of metal of mass 300g at 1000c is dropped into a logged calorimeter of heat capacity40Jk-1, containing 200g of water at 200c. The...
(Solved)
A block of metal of mass 300g at 1000c is dropped into a logged calorimeter of heat capacity40Jk-1, containing 200g of water at 200c. The temperature of the resulting mixture is 340c
Determine:
(i) Heat gained by calorimeter.
(ii) Heat gained by water.
(iii) Heat lost by the metal block.
(iv) Specific heat capacity of the metal block.
Date posted:
April 2, 2019
.
Answers (1)
-
A mass of 2kg is attached to a string of length 50 cm. It is whirled in a circle in a vertical plane
at 10 revolution...
(Solved)
A mass of 2kg is attached to a string of length 50 cm. It is whirled in a circle in a vertical plane
at 10 revolution per second about a horizontal axis. Calculate the tension in the string when the
mass is at the :-
(a)Highest point of the circle.
(b) Lowest part of the circle.
Date posted:
April 2, 2019
.
Answers (1)
-
Give a reason why bodies in circular motion undergo acceleration even when their speed is constant
(Solved)
Give a reason why bodies in circular motion undergo acceleration even when their speed is constant
Date posted:
April 2, 2019
.
Answers (1)
-
A ship of mass 1300 tonnes floats on sea water:
(Solved)
A ship of mass 1300 tonnes floats on sea water:
(i) What volume of sea water is displaced (Density of sea water is 1025kg/m3)
(ii) Suppose it sails from sea water to fresh water, what cargo must be removed so that the same volume of water is displaced? (Density of fresh water = 1000kg/m3
Date posted:
April 2, 2019
.
Answers (1)
-
A block of glass of mass 250g floats in mercury. What volume of glass lies under the surface
of Mercury? Density of mercury is 13.6 x...
(Solved)
A block of glass of mass 250g floats in mercury. What volume of glass lies under the surface
of Mercury? Density of mercury is 13.6 x 103 Kg/m3.
Date posted:
April 2, 2019
.
Answers (1)
-
The volume of a bubble at the base of a container of water is 3cm3. The depth of water is 30cm. The bubble rises up...
(Solved)
The volume of a bubble at the base of a container of water is 3cm3. The depth of water is 30cm. The bubble rises up the column until the surface ;
(i) Explain what happens to the bubble as it rises up the water column
(ii) Determine the volume of the bubble at a point 5cm below the water surface
Date posted:
April 2, 2019
.
Answers (1)
-
A column of air 5cm is trapped by mercury thread of 10cm as shown in the figure below.If the tube is laid horizontally as shown...
(Solved)
A column of air 5cm is trapped by mercury thread of 10cm as shown in the figure below.If the tube is laid horizontally as shown in (b), calculate the new length of trapped air (atmospheric pressure =75.0cmHg and density of mercury = 13600kgm-3)

Date posted:
April 2, 2019
.
Answers (1)
-
.... Three resistors of resistance 2.0 ohms, 4.0 ohms and 6.0 ohms are connected together in a circuit.
(Solved)
Three resistors of resistance 2.0 Ω, 4.0 Ω and 6.0 Ω are connected together in a circuit.
Draw a circuit diagram to show the arrangement of the resistor which Gives:
(i) Effective resistance of 3.0 Ω
(ii) Minimum resistance
Date posted:
March 27, 2019
.
Answers (1)
-
You are provided with the following; A cell and holder, a switch, a rheostat, an ammeter, a voltmeter and connecting wires. Draw a diagram for...
(Solved)
You are provided with the following; A cell and holder, a switch, a rheostat, an ammeter, a voltmeter and connecting wires. Draw a diagram for a circuit that could be used to investigate the variation of the potential difference across the cell with the current drawn from the cell.
Date posted:
March 27, 2019
.
Answers (1)
-
Figure below shows three point sources of light with an opaque object placed between them and the screen.
(Solved)
Figure below shows three point sources of light with an opaque object placed between them and the screen.

Explain the nature of the shadow formed along B and C.
Date posted:
March 27, 2019
.
Answers (1)
-
The strip placed n a pivot and kept in equilibrium by forces as shown in figure below
(Solved)
The strip placed n a pivot and kept in equilibrium by forces as shown in figure below

(i) Determine the value of F and R
(ii) X is the distance from the end of the plank to the point of application of force F. Force F is now applied at various points nearer to the pivot so that x increases. Equilibrium is maintained all the time. On the axes provided sketch the relation between force F and x.

(iii) Give a reason for the answer in (ii) above
Date posted:
March 27, 2019
.
Answers (1)
-
A machine of velocity ratio 45, overcomes a load of 4.5 x 103N when an effort of 135N is applied.
(Solved)
A machine of velocity ratio 45, overcomes a load of 4.5 x 103N when an effort of 135N is applied.
Determine:
(i) The mechanical advantage of the machine
(ii) Efficiency of the machine
(iii) The percentage of the work that goes to waste.
Date posted:
March 27, 2019
.
Answers (1)
-
Figure below shows part of a hydraulic press. The plunger is the position where effort is applied while the Ram piston is the position where...
(Solved)
Figure below shows part of a hydraulic press. The plunger is the position where effort is applied while the Ram piston is the position where load is applied. The plunger has cross-section area, a m2 while the Ram piston has cross section area, a m2.

When the plunger moves down a distance d the Ram piston moves up a distance D.
(i) State the property of liquid pressure on which the working of the hydraulic press works.
(ii) Derive an impression for the velocity ratio (V.R) in terms of A and a.
Date posted:
March 27, 2019
.
Answers (1)
-
The masses of equal volumes of a certain liquid and of water were found to be mv and mw respectively. Given that the density of...
(Solved)
The masses of equal volumes of a certain liquid and of water were found to be mv and mw respectively. Given that the density of water is 1gcm-3, express the density, p, of the liquid in terms of mv mw (show your work)
Date posted:
March 27, 2019
.
Answers (1)
-
A radio repairer wishes to use an ammeter to detect a faulty diode. With the aid of a circuit diagram describe how he will go...
(Solved)
A radio repairer wishes to use an ammeter to detect a faulty diode. With the aid of a circuit diagram describe how he will go about this task.
Date posted:
March 27, 2019
.
Answers (1)