- Draw the cross-section of a basic solar heating panel that uses heat from the sun to warm
water which flows through pipes(Solved)
Draw the cross-section of a basic solar heating panel that uses heat from the sun to warm
water which flows through pipes
Date posted: April 2, 2019. Answers (1)
- Distinguish between natural and forced convection currents.(Solved)
Distinguish between natural and forced convection currents.
Date posted: April 2, 2019. Answers (1)
- The total weight of a car with passengers is 25,000N. The area of contact of each of the four
tyres with the ground is 0.025m2. Determine...(Solved)
The total weight of a car with passengers is 25,000N. The area of contact of each of the four
tyres with the ground is 0.025m2. Determine the minimum car tyre pressure
Date posted: April 2, 2019. Answers (1)
- A block measuring 20cm x 10cm by 5cm rests on a flat surface. The block has a weight of 3N.
Determine the maximum pressure it exerts...(Solved)
A block measuring 20cm x 10cm by 5cm rests on a flat surface. The block has a weight of 3N.
Determine the maximum pressure it exerts on the surface.
Date posted: April 2, 2019. Answers (1)
- The figure shows a liquid in a pail.(Solved)
The figure shows a liquid in a pail.

Suggest a reason why pail manufacturers prefer the shape shown to other shapes
Date posted: April 2, 2019. Answers (1)
- Calculate the pressure of the gas supply.(Solved)
Calculate the pressure of the gas supply.
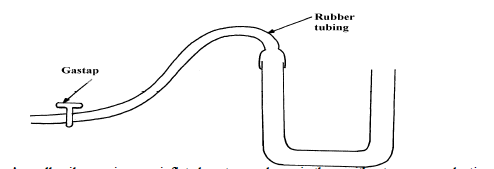
Date posted: April 2, 2019. Answers (1)
- Two immiscible liquids are poured in a container to the levels shown in the diagram below.(Solved)
Two immiscible liquids are poured in a container to the levels shown in the diagram below.

If the densities of the liquids A and B are 1g/cm3 and 0.8g/cm3 respectively, find the pressure
acting upon solid C at the bottom of the container due to the liquids
Date posted: April 2, 2019. Answers (1)
- A helical spring extends by 1 cm when a force of 1.5N is applied to it. Find the elastic potential
energy stored in it.(Solved)
A helical spring extends by 1 cm when a force of 1.5N is applied to it. Find the elastic potential
energy stored in it.
Date posted: April 2, 2019. Answers (1)
- Figure below shows a measuring cylinder of height 30cm filled to a height of 20cm with water and the rest occupied by kerosene.(Solved)
Figure below shows a measuring cylinder of height 30cm filled to a height of 20cm with water and the rest occupied by kerosene.
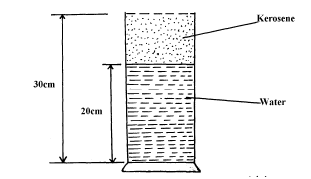
Given that density of water = 1000Kgm-3, density of kerosene = 800Kgm-3 and atmospheric pressure = 1.03x105 pascals, determine the pressure acting on the base of the container.
Date posted: April 2, 2019. Answers (1)
- The barometric height at sea level is 76cm of mercury while at a point on a highland it is 74cm of mercury. What is the...(Solved)
The barometric height at sea level is 76cm of mercury while at a point on a highland it is 74cm of mercury. What is the altitude of the point? (Take g = 10m/s2 , density of mercury =
13600kg/m3 and density of air as 1.25kg/m3)
Date posted: April 2, 2019. Answers (1)
- The figure below shows to light pith balls arranged as shown.(Solved)
The figure below shows to light pith balls arranged as shown.
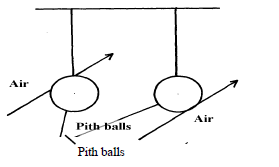
State what is observed when air is blown on the outer sides of the pith balls.
Date posted: April 2, 2019. Answers (1)
- What is the density of alcohol?(Solved)
What is the density of alcohol?
Date posted: April 2, 2019. Answers (1)
- State the definition of atmospheric pressure(Solved)
State the definition of atmospheric pressure
Date posted: April 2, 2019. Answers (1)
- State one factor that determines the depth to which mercury is depressed in a glass capillary tube.(Solved)
State one factor that determines the depth to which mercury is depressed in a glass capillary tube.
Date posted: April 2, 2019. Answers (1)
- A bathroom shower has 200 holes each 2.5mm2 in area. Water flows from a pipe of cross-section
area of 15cm at 5m/s to the shower. Determine...(Solved)
A bathroom shower has 200 holes each 2.5mm2 in area. Water flows from a pipe of cross-section
area of 15cm at 5m/s to the shower. Determine the speed of the spray.
Date posted: April 2, 2019. Answers (1)
- A piece of metal N of mass 2kg weighs 18N in water and 12N in liquid M. Determine the density of ;(Solved)
A piece of metal N of mass 2kg weighs 18N in water and 12N in liquid M. Determine the density of ;
(i) The metal N
(ii) The liquid M
Date posted: April 2, 2019. Answers (1)
- A measuring cylinder contains 50cm3 of light oil at 0oC. When a lump of dried ice is placed in the oil, the total volume is...(Solved)
A measuring cylinder contains 50cm3 of light oil at 0oC. When a lump of dried ice is placed in the oil, the total volume is 72cm3. Determine the density of the ice.
Date posted: April 2, 2019. Answers (1)
- The figure below shows a balloon carrying hydrogen gas 3m3 of density 0.09kgm-3. The mass of the balloon fabric is 2kg and the density of...(Solved)
The figure below shows a balloon carrying hydrogen gas 3m3 of density 0.09kgm-3. The mass of the balloon fabric is 2kg and the density of air is 1.25kgm-3
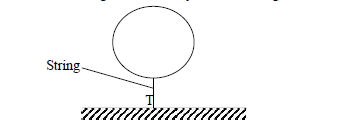
i) Determine the tension in the string
ii) If the string is suddenly cut, calculate the acceleration of the balloon upwards
iii) What is the maximum mass of the equipment the balloon can lift at a constant velocity
Date posted: April 2, 2019. Answers (1)
- A block of mass 5kg rests on an inclined surface as shown in the diagram below:(Solved)
A block of mass 5kg rests on an inclined surface as shown in the diagram below:
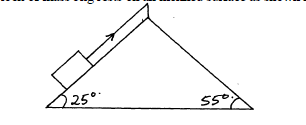
Determine the static friction on the block
Date posted: April 2, 2019. Answers (1)
- Give an example where force is applied and no work is done(Solved)
Give an example where force is applied and no work is done
Date posted: April 2, 2019. Answers (1)