Mass of density bottle when empty =20g when full of water =45g mass of water=(45-20)g=25g but density of water is always=1g/cm3 therefore 25g of water will occupy 25cm3 volume volume of density bottle =25cm3 mass of mercury=(360-20)g=340g density =mass/volume density of mercury= (340/25) =13.6g/cm3
Mzitoo answered the question on May 17, 2019 at 00:58
- Derive the three equations of linear motion.(Solved)
Derive the three equations of linear motion.
Date posted: April 23, 2019. Answers (1)
- The figure below represents part of a tape pulled through a ticker-timer by a trolley moving down an inclined plane. If the frequency of the...(Solved)
The figure below represents part of a tape pulled through a ticker-timer by a trolley moving down an inclined plane. If the frequency of the ticker-timer is 50Hz, calculate the acceleration of the trolley.

Date posted: April 23, 2019. Answers (1)
- A paper tape was attached to a moving trolley and allowed to run through a ticker-timer. The figure below shows a section of the tape.(Solved)
A paper tape was attached to a moving trolley and allowed to run through a ticker-timer. The figure below shows a section of the tape.

If the frequency of the ticker-timer is 20Hz, determine:
a) The velocity between AB and CD.
b) The acceleration of the trolley.
Date posted: April 23, 2019. Answers (1)
- A body is made to change its velocity from 20m/s to 36m/s in 0.1s. What is the acceleration produced?(Solved)
A body is made to change its velocity from 20m/s to 36m/s in 0.1s. What is the acceleration produced?
Date posted: April 23, 2019. Answers (1)
- Outline the three common types of motion:(Solved)
Outline the three common types of motion:
Date posted: April 23, 2019. Answers (1)
- A machine with a velocity ratio of 30 moves a load of 300N when an effort of 200N is applied. Calculate the efficiency of the...(Solved)
A machine with a velocity ratio of 30 moves a load of 300N when an effort of 200N is applied. Calculate the efficiency of the machine.
Date posted: April 22, 2019. Answers (1)
- Explain why a man using a parachute falls through air slowly while a stone falls through air very fast(Solved)
Explain why a man using a parachute falls through air slowly while a stone falls through air very fast.
Date posted: April 22, 2019. Answers (1)
- A man makes a deeper marks while walking on a soft ground in a high heeled shoes than in a flat shoes. Explain(Solved)
A man makes a deeper marks while walking on a soft ground in a high heeled shoes than in a flat shoes. Explain
Date posted: April 22, 2019. Answers (1)
- Explain how temperature affects Brownian motion(Solved)
Explain how temperature affects Brownian motion.
Date posted: April 18, 2019. Answers (1)
- State the applications of extended light sources(Solved)
State the applications of extended light sources
Date posted: April 15, 2019. Answers (1)
- State the advantage of the Pinhole Camera over the Lens Camera(Solved)
State the advantage of the Pinhole Camera over the Lens Camera
Date posted: April 15, 2019. Answers (1)
- Discuss the Maintenance of the Accumulators(Solved)
Discuss the Maintenance of the Accumulators
Date posted: April 15, 2019. Answers (1)
- Define Electromotive Force (E.m.f) and Potential Difference(Solved)
Define Electromotive Force (E.m.f) and Potential Difference
Date posted: April 15, 2019. Answers (1)
- What is the danger of Electrostatics?(Solved)
What is the danger of Electrostatics?
Date posted: April 15, 2019. Answers (1)
- Explain the applications of Thermal Radiation(Solved)
Explain the applications of Thermal Radiation
Date posted: April 15, 2019. Answers (1)
- Discuss lagging and give an example of its application(Solved)
Discuss lagging and give an example of its application.
Date posted: April 15, 2019. Answers (1)
- Explain the mode of Operation of the Six’s Thermometer(Solved)
Explain the mode of Operation of the Six’s Thermometer
Date posted: April 15, 2019. Answers (1)
- Give the comparison between Mercury and Alcohol as Thermometric Liquids(Solved)
Give the comparison between Mercury and Alcohol as Thermometric Liquids
Date posted: April 15, 2019. Answers (1)
- The height, h of a water manometer is 20 cm when used to measure pressure of a gas.(Solved)
The height, h of a water manometer is 20 cm when used to measure pressure of a gas.
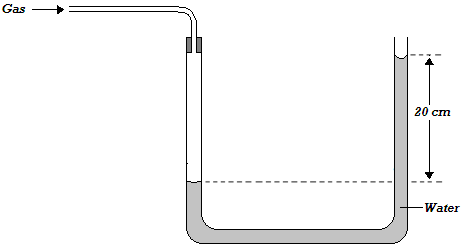
a) Determine the pressure due to gas, If atmospheric pressure is 103000N/m2.
b) What would be the height if the liquid used is glycerin of density 1.26g/cm3
Date posted: April 15, 2019. Answers (1)
- A sphere of diameter 6.0 mm is molded into a uniform wire of diameter 0.2mm. Calculate the length of the wire.(Solved)
A sphere of diameter 6.0 mm is molded into a uniform wire of diameter 0.2mm. Calculate the length of the wire.
Date posted: April 15, 2019. Answers (1)